News
ATAGI approves fourth dose for severely immunocompromised
Described as a booster – rather than part of the primary vaccination course – people are eligible four months after their third dose.
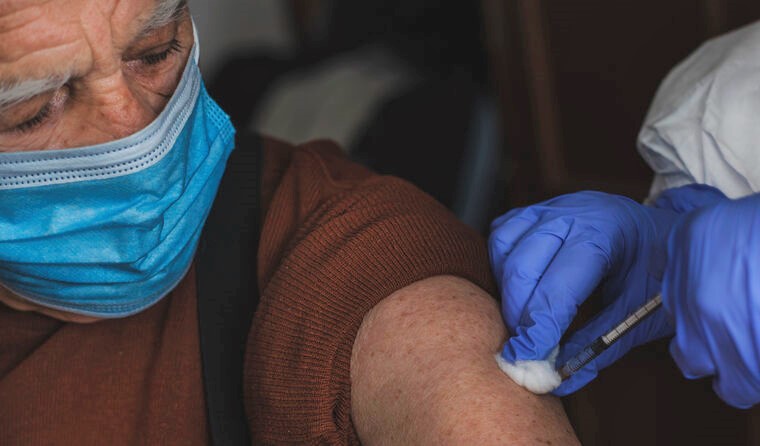 The DoH says the change will bring access to boosters for the immunocompromised into the same pattern as for the broader population.
The DoH says the change will bring access to boosters for the immunocompromised into the same pattern as for the broader population.
The Australian Technical Advisory Group on Immunisation (ATAGI) has approved a fourth dose of a COVID-19 vaccine for people with weakened immune systems.
The fourth dose is being described as a booster rather than part of the primary vaccination course, with people eligible to receive it four months after their third dose.
A Department of Health statement issued on Christmas Eve says the change will bring access to boosters for the immunocompromised into the same pattern as for the broader population.
‘Immunocompromised individuals who have received three primary doses of a COVID-19 vaccine are also recommended to have a booster dose in line with the timing for the general population, ie currently a four-month interval from their primary course and, when capacity permits, three months,’ the statement reads.
The change to the existing arrangement was made just before Christmas, with the emergence of the highly transmissible Omicron variant of concern cited as a reason.
‘There are no data to support the use of any additional primary doses of COVID-19 vaccine after a third primary dose,’ the ATAGI advice reads.
‘Patients who do not respond to third doses may not respond to subsequent doses.
‘However, due to the current outbreak with the Omicron variant of concern, ATAGI recommends that immunocompromised individuals who have received three primary doses of a COVID-19 vaccine have a booster dose at four months, in line with the timing for the general population.’
In early October, ATAGI recommended a third vaccine dose as part of a primary vaccination course for those with severely weakened immune systems.
The Pfizer and Moderna mRNA vaccines are stated as the preferred vaccine for a third primary course dose. Both have also been approved for use as boosters.
For the third primary course dose, ATAGI says AstraZeneca can still be used for patients who had it for their first two doses without any contraindications, or if they have had a significant adverse reaction after a previous mRNA vaccine dose.
The ATAGI advice states that those who are not severely immunocompromised but are due for significant immunosuppressive therapy more than two weeks after their second dose do not require a third dose as part of their primary vaccination course.
The group also advises those with severe immunocompromise who have had more than six months’ interval since their second dose to receive a third dose ‘as soon as feasible’.
While the third dose is recommended between two and six months after a second dose, ATAGI states that a shorter interval of four weeks could be considered ‘in exceptional circumstances’, including ‘anticipated intensification of immunosuppression’, as well as outbreaks.
ATAGI states that the recommendations were prepared following consultation with the Australasian Society of Clinical Immunology and Allergy (ASCIA).
The full ATAGI recommendation is available online.
Log in below to join the conversation.
boosters COVID-19 immunocompromise vaccination
newsGP weekly poll
Which RACGP request would you most like the Government to fund in the upcoming Federal Budget?