Author guidelines
Download author guidelines
Download standards for reporting qualitative research (SRQR)
Download SRQR checklist
Last revision: September 2020
1. About the journal
The Australian Journal of General Practice (AJGP) aims to provide relevant, evidence-based, clearly articulated information to Australian general practitioners (GPs) to assist them in providing the highest quality patient care, applicable to the varied geographic and social contexts in which GPs work and to all GP roles as clinician, researcher, educator, practice team member and opinion leader. All articles are subject to peer review before they are accepted for publication. The journal is indexed in MEDLINE, Index Medicus and Science Citation Index Expanded.
The Journal was established in 1956 as the Annals of General Practice. In 1971 the Journal was retitled Australian Family Physician (AFP). In 2018 the Journal was renamed Australian Journal of General Practice (AJGP).
AJGP is a member, and subscribes to the principles, of the Committee on Publication Ethics (COPE), and to those of the International Committee of Medical Journal Editors (ICMJE).
All submitted articles are subject to a peer-review process before they are accepted for publication. AJGP is indexed in MEDLINE, Index Medicus and Science Citation Index Expanded.
AJGP does not charge author, editorial or publication fees. All articles are available online free of charge. In addition, Fellows and members of the Royal Australian College of General Practitioners (RACGP) receive a published hard copy of each issue.
2. Manuscript categories and requirements
Manuscript requirements for accepted categories are outlined below. On application from the author in the covering letter, the editor may consider a variation of these requirements.
2.1 Clinical articles
- Description – Evidence-based review articles relating to the assessment and/or management of specific clinical problems relevant to general practice
- Structure – Introduction, aim, body, conclusion (summary of critical issues including demonstrating relevance to general practice)
- Word limit – 1500 words maximum, excluding abstract, tables, boxes, figures and references
- Abstract – 150 words maximum, structured under the headings: Background, Objective, Discussion
- References – 40 maximum
- Figures/tables/images – 5 maximum
- Key points – 5 salient points, each one line maximum in length
2.2 Clinical case studies
- Description – Clinical cases seen by the authors that demonstrate important principles of general practice. AJGP is unlikely to publish cases describing rare conditions, or cases that have been mismanaged. We encourage the patient’s perspective to be included.
- Structure – Must be presented as a short description of the clinical presentation followed by a series of relevant questions and their answers; please see published examples on the AJGP website
- Word limit – 750 words maximum, excluding tables, boxes, figures and references
- Abstract – nil
- References – 10 maximum
- Figures/tables/images – 5 maximum
- Key points – 3 salient learning points, each one line maximum in length
2.3 Professional articles
- Description – Evidence-based review articles relating to non-clinical topics relevant to general practice (eg practice management, medico-legal issues, medical ethics, patient safety, healthcare systems and service delivery, clinical workforce and medical education)
- Structure – Introduction, aim, body, conclusion (summary of critical issues including demonstrating relevance to general practice)
- Word limit – 1500 words maximum, excluding abstract, tables, boxes, figures and references.
- Abstract – 150 words maximum, structured under the headings: Background, Objective, Discussion
- References – 40 maximum
- Figures/tables/images – 5 maximum
- Key points – 5 salient points, each one line maximum in length
2.4 Viewpoint articles
- Description – Clearly argued opinion, supported by appropriate references, discussing an important and relevant topic for general practice. The article must be presented in a scholarly framework.
- Word limit – 750 words maximum; excluding tables, boxes, figures and references
- Abstract – nil
- References – 10 maximum
- Figures/tables/images – 2 maximum
2.5 Letters to the editor
- Description – A brief discussion considering a recently published article in AJGP or a current, important and relevant topic for general practice. A short report on a research topic or study that might include a pilot study, preliminary findings, or otherwise be best suited to a shorter communication. The article must be presented in a scholarly framework.
- Word limit – 350 words maximum; excluding references
- Abstract – nil
- References – 5 maximum
2.6 Research articles
The scope for research articles may include quantitative, qualitative, protocols, systematic reviews and meta-analyses. Although mixed method studies will be considered, the relatively low word count may be challenging when ensuring that the methodology and results are fully described.
- Description – Reports of high-quality, original research relevant to general practice
- Word limit – Qualitative and mixed methods research articles 3000 words maximum; all others 2000 words maximum, excluding abstract, tables, boxes, figures, checklist and references
- Abstract – 150 words maximum, structured under the headings: Background and objective, Methods, Results, Discussion
- Structure – IMRAD format is required. This includes a clear statement of the aim/hypothesis in the last paragraph of the introduction; clear, evidence based and referenced documentation of the methodology; HREC approval confirmation and document number in the last paragraph of the methods; a review of the limitations at the end of the discussion and a concluding statement that matches the scope of the work.
- References – 30 maximum
- Figures/tables/images – 5 maximum
- Human Research Ethics Committee (HREC) documentation – mandatory requirement except for systematic reviews and meta-analyses
- Clinical trials – all articles reporting any form of clinical trial must include the registration number with the Australian and New Zealand Clinical Trials Registry.
2.6.1 Qualitative research
- All qualitative research submissions to AJGP require a formal report addressing the Standards for Reporting Qualitative Research (SRQR) at the time of submission.
- The 21-item standard is outlined at http://www.equator-network.org/reporting-guidelines/srqr/ and the SRQR checklist is available to download.
- Provide your response to each of the 21 standards in a four-column table, with the first column being the standard number, the second being the description of the standard, the third a brief description of how you have met the standard or how the standard does not apply and the fourth column noting the page and line number in the manuscript where the standard has been detailed.
- The document tabulating your response to each of the standards is to be uploaded as a stand-alone document and not to be included in other documentation or the manuscript.
- Please note that AJGP may elect to publish your tabular response.
2.6.2 Protocol research
- Protocol research submissions require a plan for a proposed research study, outlining the objectives of the study, hypotheses to be tested, and methodology and analyses to be used. Authors must clearly explain why their protocol is sufficiently important, novel or ground-breaking to justify publication. HREC approval must be demonstrated where applicable.
2.6.3 Questionnaire research
- All questionnaire research submissions to AJGP require a formal report addressing the ‘Critical appraisal checklist for a questionnaire study’ at the time of submission.
- The ‘Critical appraisal checklist for a questionnaire study’ is noted as ‘Table E’ located online at www.bmj.com/content/328/7451/1312/related#e (from Boynton Petra M, Greenhalgh T. Selecting, designing, and developing your questionnaire BMJ 2004;328:1312. doi: 10.1136/bmj.328.7451.1312 )
- Authors should provide a response to each of the standards in a four-column table, with the first column being the standard number, the second being the description of the standard, the third a brief description of how the standard has been met or how the standard does not apply and the fourth column noting the page and line number in the manuscript where the standard has been detailed.
- The document tabulating the authors’ response to each of the standards is to be uploaded as a standalone document and not to be included in other documentation or the manuscript.
- Please note that AJGP may elect to publish authors’ tabular responses.
3. Manuscript style
3.1 Manuscript title
The title length is limited to 20 words, to ensure focus and brevity. Please do not include headers, footers or footnotes.
3.2 Manuscript text
Manuscripts should follow internationally accepted, scholarly academic style. In most circumstances, write in the third person, past tense.
This includes:
- use generic names when referring to medicines; do not use brand or trade names, do not capitalise and check spelling to ensure correct to avoid misinterpretation
- use acronyms and abbreviations sparingly; spell out all acronyms and abbreviations in full at the first reference
- clearly indicate headings and subheadings; do not use all capitals in headings
- label all tables, boxes and figures and include references to the tables, boxes and figures in numerical order in the text
- submit tables, boxes and figures in an editable form in portrait orientation not exceeding one page per table, box or figure
- ensure all medical claims and statements are referenced
- ensure informed consent is obtained from patients or their legal representative for any patient material discussed or depicted in any manuscript submitted to AJGP. The RACGP’s ‘Patient consent’ form provides a template for ensuring informed consent and must be completed and retained by the author and the patient or their legal representative. Authors must not supply these forms to AJGP unless directed. This requirement applies whether or not clinical photographs are used. Forms are available on the ScholarOne Manuscripts website. Submissions will not progress until confirmation of patient or legal representative consent is attained through provision of a signed ‘Assignment of copyright and Health information authorisation’ form.
- all patient information must be de-identified.
3.3 Tables, boxes and figures
Tables, boxes, images, figures and illustrations are used to support the text without duplicating its content. Each must be numbered and have an in-text reference and a caption. Tables, boxes and figures must not exceed one A4 page in portrait orientation at 8 point standard font. Tables, boxes and figures should be supplied in an editable file format so they can be formatted correctly for publication.
Graphs, flowcharts and algorithms should, preferably, be supplied in their native form (eg Microsoft Excel). If native files cannot be supplied, please provide numeric data for graphs in the event they need to be redrawn. Images that include the author’s own text should be supplied in an editable format.
A copy of written permission from the copyright holder, if required, must be provided for all tables, boxes, images, figures or illustrations that have been reproduced or adapted from copyrighted sources, including online material (refer to Section 4.4 ‘Permissions’ for further details).
3.4 References
AJGP uses Vancouver referencing style. Please submit articles referenced in standard Vancouver style.
Articles in preparation or submitted for publication must not be included in the reference list, but can be mentioned in the text as ‘unpublished data’ with a list of authors (or initials if the authors are co-authors of the present work). Written permission from the authors of any unpublished data or personal communications must be provided.
Linked reference fields (eg EndNote codes) must be removed before manuscript submission. In-text reference numbers should be superscript and in numerical order. Numbering of additional references cited in tables, boxes and figures should continue from the last reference in the main body of the article, if not already cited. For example, if the last reference in the article is number 20, any additional references in tables, boxes and figures should continue as 21, 22, etc, if not already cited in the main body of the article.
4. Manuscript submission
All manuscripts are submitted in electronic format via the ScholarOne manuscript website portal (http://mc.manuscriptcentral.com/ajgp).
4.1 Title page
Upload the title page as a separate document in Scholar One; this helps facilitate the peer review process.
The title page
must contain:
- the title of the article
- a list of all authors in publication order and each author’s formal qualifications (postnominals), current position(s) and affiliation(s)
- contact details of the designated corresponding author
- competing interest statement (refer to Section 4.6)
- funding statement (refer to Section 4.6)
- acknowledgements, if applicable
- word count (excluding abstract, tables, boxes, figures and references).
4.2 Covering letter
The covering letter is a direct communication to the editor and provides a unique opportunity to demonstrate succinctly why the article is best published in AJGP.
4.3 Copyright
Authors must complete a standard ‘Assignment of copyright and Health information authorisation’ form. This can be found on the Scholar One website.
4.4 Permissions
On the ‘Assignment of copyright and Health information authorisation’ form, authors must indicate whether tables, boxes and figures:
- are original creations
- contain information taken from published sources
- are reproduced or adapted from published sources.
Authors are responsible for obtaining permission from the appropriate copyright holder to reproduce or adapt all published (or otherwise copyright) material. The copyright holder’s permission statement must be provided at the time of manuscript submission, with a copy of the source material. Refer to Section 8 for information about permission to reproduce content from an AJGP article.
4.5 Author contributions
Authors must meet all of the following four criteria, as outlined in the ICMJE recommendations:
- Substantial contributions to the conception or design of the work; or the acquisition, analysis or interpretation of data for the work; AND
- Drafting the work or revising it critically for important intellectual content; AND
- Final approval of the version to be published; AND
- Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Author contribution statements and acknowledgements in research papers should state clearly and specifically whether, and to what extent, the authors used AI technology, such as ChatGPT, in the preparation of their manuscript and analysis. The authors should also indicate which large language models were used.
Ghost authorship is not permitted by AJGP. All contributors who fulfil the ICMJE criteria for authorship must be listed as authors.
Individuals who have assisted with writing the manuscript (eg medical writers, professional editors) must be named in an acknowledgement section, and any competing interests they have must be declared (refer to Section 4.6).
Other contributors who do not meet authorship requirements can be included in an acknowledgement section and their contribution specified. Permission to acknowledge is required from those who are named. The contribution of each listed author must be outlined in the cover letter.
4.6 Disclosure of funding arrangements and/or competing interests
Any potential conflicts of interest must be stated on the ‘ICMJE form for disclosure of potential conflicts of interest’ available on the Scholar One Manuscripts site.
Declarations on funding and financial arrangements must also be made in sections 2 and 3 of the ICMJE form.
Authors must also include a ‘Competing interests’ statement in their cover letter. The statement should specify board memberships of, affiliations with, and/or funding/honoraria from pharmaceutical or other for-profit organisations. Sponsorship or funding arrangements relating to the authors’ work must be disclosed.
If a commercial organisation has initiated or significantly contributed to the writing of the article, the organisation must be identified. The ‘Competing interests’ and funding statements must include any interests and potential conflicts identified in the ICMJE form. If there are no competing interests and no funding, authors should nevertheless include a statement of ‘None’.
When competing interests and funding are indicated as present, authors must include in their covering letter the following statement: ‘I/we had full access to all relevant data in this study, and supporting sources had no involvement in data analysis and interpretation, or in the writing of the article’.
While declared competing interests and funding do not automatically exclude an article from publication, articles with declarations other than ‘none’ may be referred to the AJGP Editorial Board to further scrutinise whether such competing interests and funding might have compromised the integrity of the article.
Articles that have been commissioned or funded by a commercial company or organisation may be rejected if the editors perceive that content has been unduly influenced by the interests of the commissioning or funding company or organisation. This includes articles written by paid employees of commercial companies or organisations.
5. Peer review and acceptance
Acceptance of manuscripts for publication is based on quality, originality and relevance for a general practitioner readership.
Articles submitted to AJGP are subject to peer review, which may consist of more than one round.
AJGP editors make the final decisions on acceptance or rejection. The editors reserve the right to reject any manuscript without peer review if it is not considered relevant to general practice or is otherwise unsuitable for publication in AJGP.
AJGP reserves the right to use plagiarism detection software. By submitting a manuscript, the authors accept that it may be screened against previously published works. AJGP processes are informed by the COPE guidelines for managing cases of suspected plagiarism (http://publicationethics.org/resources/flowcharts).
AJGP publication process (including peer review)
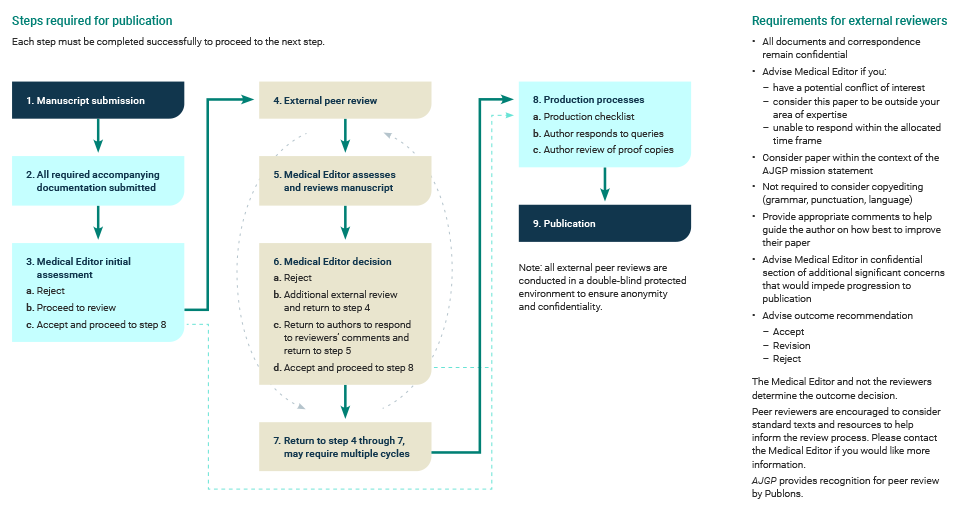
Click here to enlarge
6. Responding to peer reviewers’ comments
Authors must provide a point-by-point explanation detailing how they have responded to each of the peer reviewers’ comments in a table with each item numbered. The revised manuscript must be submitted as a ‘track changes’ version, plus a ‘clean copy’ version with all changes accepted.
7. Post-acceptance
All accepted manuscripts are subject to editing for length, clarity and conformity with AJGP style.
A PDF proof of the final manuscript of accepted articles will usually be sent to the corresponding author before publication and must be returned by the date requested.
It is the author’s responsibility to carefully check the final proof and ensure that no mistakes have inadvertently occurred in the production process, especially in regard to numbers and statistics in tables, or investigation results and medication doses.
Please note that AJGP reserves the right to rescind or withdraw any manuscript at any time, including after publication, if undeclared concerns with authorship, conflicts of interest, plagiarism or content veracity and accuracy arise.
7.1 Appeals
The AJGP complaints and appeals process is informed by the COPE guidelines. In the first instance, concerns should be directed to the Editor in Chief at AJGP@racgp.org.au
8. Permissions post-publication
Permission to reproduce all or part of any AJGP article must be obtained by emailing permissions@racgp.org.au
Further details are available at https://www1.racgp.org.au/ajgp/about-the-journal