Difficult-to-treat asthma
Most people’s asthma can be effectively managed with currently available medicines; however, a significant subset of people still have uncontrolled asthma despite treatment (Box 1).1 Difficult-to-treat asthma is asthma that remains uncontrolled despite treatment with high-dose inhaled corticosteroids/long-acting bronchodilators (ICS/LABA) and/or systemic corticosteroids, or asthma that requires such medication to remain well controlled.1 High-dose ICS is defined as doses >800 µg of budesonide or equivalent per day.2 For these patients, poor asthma control is most commonly due to factors other than the asthma itself, including suboptimal adherence to treatment or incorrect inhaler technique.3 It is estimated that 17% of people with asthma have difficult-to-treat asthma.4
| Box 1. Criteria for uncontrolled asthma, according to International European Respiratory Society/American Thoracic Society guidelines1 |
| Uncontrolled asthma is defined as at least one of the following: |
1. Poor symptom control: in the past four weeks the patient has had at least one of the following
- Daytime asthma symptoms more than twice per week
- Any night waking due to asthma
- Reliever needed for symptoms more than twice per week
- Any activity limitation due to asthma
|
| 2. Frequent severe exacerbations: two or more bursts of oral corticosteroids in the previous year |
| 3. Serious exacerbations: at least one hospitalisation, ICU admission or mechanical ventilation in the previous year |
| 4. Airflow limitation:* FEV1 <80% predicted (after appropriate bronchodilator withheld and with reduced FEV1/FVC). |
*The lung-function criterion for uncontrolled asthma is debatable.19
FEV1, forced expiratory volume in one second; FVC, forced vital capacity; ICU, intensive care unit |
Severe asthma
Severe asthma is a subset of difficult-to-treat asthma. Patients who present with difficult-to-treat asthma and fail to improve despite confirmation of the diagnosis and treatment of confounders – such as inhaler technique, adherence, risk factors, triggers and comorbidities – are categorised as having severe asthma.1 In other words, severe asthma is defined by the fact that symptoms and/or exacerbations remain uncontrolled despite addressing all potential contributory factors. While mild asthma is asthma that can be effectively controlled with reliever alone or low-dose ICS, the key characteristic of severe asthma is the requirement for high-dose ICS/LABA to control asthma, or the inability of high-dose ICS/LABA (with or without oral corticosteroids) to control asthma symptoms and/or exacerbations.1,5
Although severe asthma accounts for only 3–10% of the population of adults with asthma, the disease represents a disproportionately high burden on both the healthcare system and patients.6 Canadian data suggest that severe asthma accounts for more than 60% of the costs associated with asthma and imposes a significant burden on patients because of ongoing symptoms, exacerbations and medication side effects.7,8
The concept of severe asthma has evolved substantially over time and is commonly misunderstood in primary care. This confusion has been driven in part by the use of severity descriptors for asthma control, including intensity and frequency of symptoms, severity of exacerbations and degree of obstruction.9 One of the criticisms of these historical concepts is that they do not reflect underlying severe disease, as frequent and severe symptoms may become well controlled with optimised ICS. Therefore, asthma control and severity are not synonymous and should not be used interchangeably.5,9 Unlike many other disease states – which follow the therapeutic principle that treatment decisions should follow diagnosis and initial assessment of severity – asthma severity requires retrospective assessment of the level of treatment needed to achieve good asthma control and can only be done after several months of treatment.1,10
Goals of treatment
The long-term goals of asthma management are to:5
- achieve good symptom control and maintain normal activity levels
- minimise future risk of exacerbations, fixed airflow limitation and medication side effects.
Asthma control includes both components. To achieve these goals, current international and national guidelines suggest control-based asthma management.2,5 Some patients may not achieve these goals because of truly refractory severe disease. For others, however, difficult-to-treat asthma can be attributed to potentially modifiable factors such as inhaler technique, adherence, comorbidities, persistent environmental exposures or psychological factors.5 It is therefore important that clinicians are able to differentiate between difficult-to-treat asthma and severe asthma in order to optimise management.
A systematic approach to difficult‑to-treat asthma
A systematic approach to difficult-to-treat asthma is required to both improve patient outcomes and identify those patients who would benefit from a referral to a specialist physician experienced in the management of asthma (Figure 1).2,11
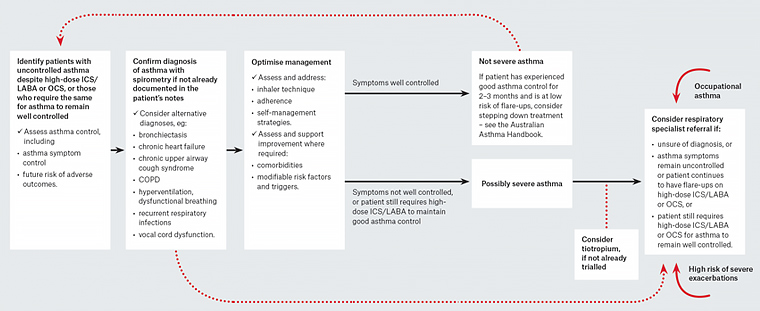
Figure 1. A systematic approach to investigating and managing adults with difficult-to-treat asthma
Reproduced with permission from NPS MedicineWise, Management of people with difficult-to-treat asthma: A systematic approach. Surry Hills, NSW: NPS MedicineWise, 2018.
COPD, chronic obstructive pulmonary disease; ICS, inhaled corticosteroid; OCS, oral corticosteroid; LABA, long-acting beta agonist
Confirm the diagnosis
Confirmation of a patient’s asthma diagnosis with objective testing (such as spirometry), if not previously documented, is an essential first step in a systematic approach to difficult-to-treat asthma. Studies show that up to a third of patients with doctor-diagnosed asthma do not have asthma when objectively measured.12 Patients with an unclear diagnosis or clinical features that suggest an alternative diagnosis should be referred promptly to a respiratory specialist.2,5
Assess patient technique and adherence
Poor adherence to inhaled corticosteroids is associated with an increased risk of poor symptom control and exacerbations, so should be assessed regularly.5 Poor adherence can be both intentional – often driven by lack of perceived necessity or concerns of adverse effects of corticosteroids – and unintentional, often arising from issues with inhaler technique, dosing regimen or cost of medicines.5 Studies have shown that poor adherence to treatment occurs in approximately half of the patients with difficult-to-treat asthma,4 and only 31% of people with asthma have optimal inhaler technique.13
Manage comorbidities, risk factors and triggers
Several comorbidities frequently present in people with difficult-to-treat and severe asthma. The presence of one or more comorbid conditions can compromise patients’ quality of life, contribute to symptom burden and lead to medicine interactions.5 Furthermore, these comorbid conditions – such as chronic rhinosinusitis, bronchiectasis and anxiety or depression and other risk factors such as exposure to tobacco smoke or allergens (Table 1) – may complicate the management of asthma and contribute to poor symptom control.2
| Table 1. Factors that may contribute to poor symptom control and/or exacerbations19 |
| Medicines and related |
Exposure |
Comorbidities |
- Incorrect inhaler technique
- Poor adherence with preventer therapy
- Medicines that may exacerbate asthma
(eg aspirin and beta-blockers)
- High short-acting bronchodilator use
|
- Respiratory viruses
- Smoking or environmental tobacco smoke; biomass fuel exposure
- Allergen exposure in sensitised patients (house dust mite, cat, mould, cockroach)
- Confirmed food allergy
- Indoor or outdoor air pollution, extreme weather
- Occupational exposure to allergens or irritants
|
- Obesity
- Anxiety, depression
- Chronic obstructive pulmonary disease
- Bronchiectasis
- Gastro-oesophageal reflux disease
- Rhinosinusitis ± nasal polyposis
- Vocal cord dysfunction
- Allergic bronchopulmonary aspergillosis
- Pregnancy
|
Patients taking high-dose ICS therapy whose asthma control does not improve with correction of these factors are considered to have severe asthma.1
Treatment options
While evidence from clinical trials and meta-analyses can guide policy, individual treatment decisions should also consider patient characteristics that may predict response, patient preferences, and practical issues including adherence, dosing regimen and ability to use the delivery device.5
Current guidelines recommend management of risk factors and comorbidities, and, if necessary, intensification of treatment to achieve good asthma control. In the past, this approach often culminated in the use of oral corticosteroids for those with poor asthma control.5 This is no longer a preferred option.5 The risks associated with long-term systemic corticosteroids are increasingly well-known, and this, combined with greater understanding of asthma pathology, has stimulated the development of targeted, biologic treatments according to inflammatory phenotype.14 Recognised inflammatory profiles in severe asthma that can be targeted with currently available biologic therapy include severe eosinophilic and severe allergic asthma. It is therefore essential to identify those patients likely to respond to biologic therapy because of the high cost of these interventions.
It is important to note that severe asthma may require specific management strategies, rather than simply a categorisation of being more than a ‘worse’ form of the milder disease. Patients with severe asthma may require referral to a specialist respiratory physician.2,11 Currently in Australia, to be eligible for Pharmaceutical Benefits Scheme (PBS)-subsidised biologic therapy, patients must be under the care of a specialist experienced in the management of severe asthma for at least six months or have been diagnosed by a multidisciplinary severe asthma clinic team. These medicines are prescribed via S100 Authority prescription in accordance with specific application criteria.15,16 After the initial three doses, ongoing treatment is usually administered in primary care, but the patient must return to the specialist every 5–6 months for authorisation of ongoing PBS-subsidised treatment and review of ongoing eligibility.2
Tiotropium
In terms of options available within the primary care setting, tiotropium via mist inhaler is now PBS-subsidised for patients with severe asthma who have had at least one severe exacerbation in the past 12 months while taking optimised therapy. A recent Cochrane review found that addition of tiotropium to combination ICS/LABA in adults with severe asthma resulted in fewer exacerbations requiring oral corticosteroids, and is likely to have benefits on lung function and asthma control.17 Tiotropium may be considered before biologic add-on therapy because of its lower cost.6
Omalizumab
Omalizumab, a humanised monoclonal anti-immunoglobulin E (IgE) antibody, is indicated as an add-on therapy for people aged ≥6 years with severe allergic asthma. Omalizumab, administered every 2–4 weeks via subcutaneous injection, binds to free IgE and attenuates IgE-mediated allergic response. Meta-analysis shows omalizumab treatment is effective in reducing asthma exacerbations and hospitalisations, and is associated with a modestly reduced dose of ICS, as well as improved symptom control and health-related quality of life.18
The most common adverse effects include hypersensitivity and injection-site reactions.19
Mepolizumab
Mepolizumab is a humanised monoclonal antibody that binds to interleukin-5 (IL-5), the major cytokine responsible for growth, differentiation, recruitment, activation and survival of eosinophils.20 It is indicated as add-on therapy for people aged ≥12 years with severe refractory eosinophilic asthma. It is administered subcutaneously once every four weeks. Meta-analysis shows a significant reduction in asthma exacerbations and hospitalisation/emergency room visits for patients administered mepolizumab versus placebo.21 Mepolizumab also has an oral-steroid sparing effect, permitting a significant reduction of oral corticosteroid (OCS) dose in patients with severe OCS-dependent eosinophilic asthma.22 Modest improvements are also seen in health-related quality of life and lung function.21
Any pre-existing helminthic infections should be treated before therapy is started, as IL-5 treatment can theoretically lead to disseminated parasitic infection.23 Acute and delayed hypersensitivity reactions have occurred following administration of mepolizumab, although this is infrequent.23
Benralizumab
Another anti-IL-5 humanised monoclonal antibody – benralizumab – binds to the IL-5 receptor itself and leads to a rapid reduction in eosinophils and basophils. Benralizumab has recently been approved for use in Australia and is now listed on the PBS for uncontrolled severe eosinophilic asthma. Meta-analyses of recent trials found a significant reduction in severe exacerbations and improved lung function21 and, for patients requiring maintenance oral corticosteroids, a significant reduction in corticosteroid dose.24 Benralizumab is also administered by subcutaneous injection with pre-filled syringes every four weeks for the first three doses and then every eight weeks.24 The most common adverse effects of benralizumab include headache, pharyngitis, arthralgia and cough.25
Montelukast
There is little evidence to support the addition of montelukast for patients already taking maximal inhaled therapy, except perhaps for patients with aspirin-exacerbated respiratory disease, so it should be reserved for use in specialist settings.2
Conclusion
Managing patients with difficult-to-treat and severe asthma requires a systematic approach to ensure correct diagnosis; evaluation of adherence and inhaler technique; and identification of comorbidities, risk factors and triggers. Patients who fail to improve despite these measures may have severe asthma and may benefit from timely referral to a specialist physician or asthma clinic. New treatment options with biologic therapies are now available for a proportion of patients with truly severe asthma.
Additional resources
Australian Asthma Handbook
Severe asthma in adults and adolescents
Centre of Excellence in Severe Asthma, www.severeasthma.org.au
Severe Asthma Toolkit
NPS MedicineWise
Difficult-to-treat and severe asthma webinar
Global Initiative for Asthma (GINA)
GINA pocket guide for difficult-to-treat and severe asthma in adults and adolescents: Diagnosis and management, www.ginasthma.org