In some individuals, acute pain persists and develops into a chronic pain state, defined as daily pain lasting >3 months.1 Chronic pain is difficult to treat and can adversely affect quality of life and day-to-day function. Suicidal behaviour is 2–3 times higher in patients with chronic pain, and approximately 40% of forced early workforce retirements are due to chronic pain. One in five Australian adults are estimated to live with chronic pain, costing the community over $140 billion per annum.1,2 Chronic pain is a frequent presentation in general practice and central to commonly treated conditions such as arthritis, fibromyalgia, cancer and diabetes.
Chronic pain can be divided into three mechanistic categories: nociceptive pain maintained by constant activation of pain receptors (nociceptors); neuropathic pain that results from lesions, disease or dysfunction of the nervous system with or without peripheral nerve changes; and nociplastic pain that results from nervous system sensitisation without clear evidence of tissue or nerve damage (Figure 1).3 Current interventions for chronic pain may vary somewhat according to the type of pain observed (eg opioids for nociceptive pain, and gabapentinoids or antidepressants for neuropathic pain). Current pharmacological interventions tend to be of limited efficacy, with fewer than 20% of individuals reporting pain relief of 50% or more.4 Even when pain relief is obtained, current medications can have significant side effects – including the potential for abuse/misuse – and are often unsuitable for long-term use.5
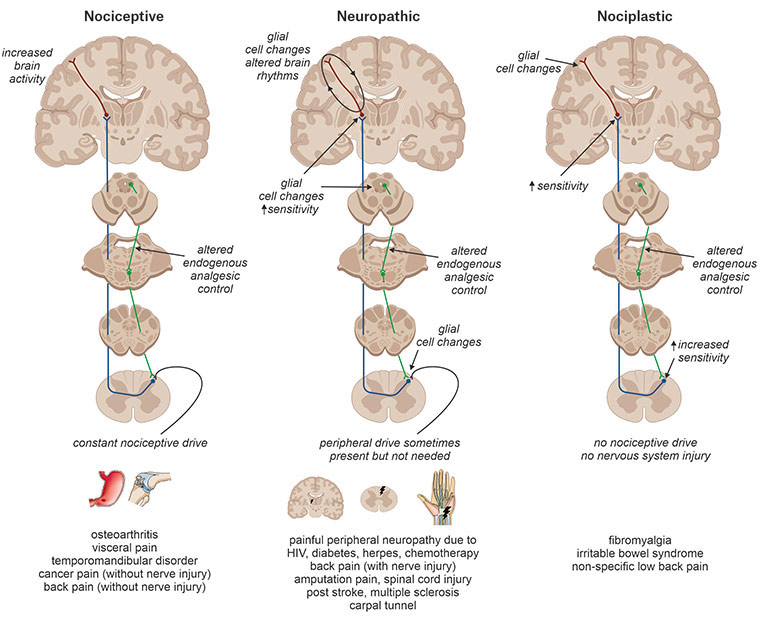
Figure 1. Chronic pain can be divided into three major categories: nociceptive, neuropathic and nociplastic. Chronic nociceptive pain is characterised by the presumed constant activation of peripheral nociceptors with limited changes in central nervous system function. Chronic neuropathic pain is characterised by damage or presumed damage to the nervous system; it is is characterised by significant changes within the central nervous system and can exist without peripheral input. Chronic nociplastic pain results from increased nervous system sensitisation with no clear evidence of actual or threatened tissue damage and without nerve injury.
HIV, human immunodeficiency virus
It is estimated that 600,000 Australians currently self-medicate with cannabis,6 with chronic pain a leading indication for such use. Most of this self-medication involves illicit cannabis,7 although a growing number of patients are now transitioning to prescribed medicinal cannabis products. Since late 2016, medicinal cannabis products can be legally prescribed by Australian doctors under the Special Access Scheme Category B (SAS-B) and the Authorised Prescriber Scheme of the Therapeutic Goods Administration (TGA). Most current prescriptions occur under the SAS-B scheme and involve doctors applying on behalf of individual patients to access a medicinal cannabis product.8–10 As of July 2021, >130,000 such approvals had been issued under SAS-B, with approximately 65% of these to treat chronic pain. After a very slow start in 2017–2019, approvals reached approximately 10,000 per month during early 2021 (Figure 2). Queensland has by far the highest per capita rate of SAS-B approvals, followed by Victoria and NSW (Table 1). Such ‘approvals’ are not necessarily clinical endorsements of medicinal cannabis; they simply indicate that TGA regulatory requirements have been met.
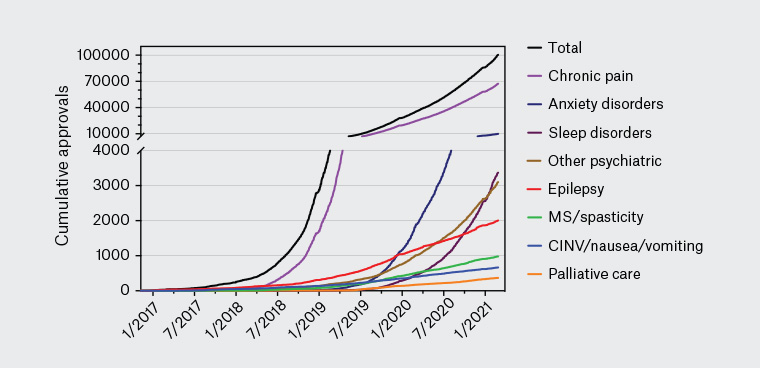
Figure 2. Recent medicinal cannabis approvals in Australia. Medicinal cannabis approvals in Australia from January 2017 – April 2021, sourced through a Freedom of Information request #2275 to the Therapeutic Goods Administration. The approval rate for products to treat various chronic pain conditions is increasing dramatically. This increase highlights the growing incidence of chronic pain experienced by Australians,1 increase in patient demand for medicinal cannabis and the possible lack of alternative effective treatment options for chronic pain. There is an urgent need for clinical trials to assess the efficacy of medicinal cannabis for chronic pain relief.
CINV, chemotherapy-induced nausea and vomiting; MS, multiple sclerosis
| Table 1. Total and chronic pain Special Access Scheme Category B (SAS-B) approvals (month of April 2021) by state/territory |
| State/territory |
All approvals |
Chronic pain approvals |
Chronic pain approvals (%) |
Population* |
Approvals
per capita† |
| Queensland |
4,006 |
2,494 |
62.3 |
5,194,900 |
77.11 |
| Victoria |
1,387 |
882 |
63.6 |
6,661,700 |
20.82 |
| New South Wales |
1,063 |
733 |
69.0 |
8,172,500 |
13.01 |
| Western Australia |
377 |
250 |
66.3 |
2,670,200 |
14.12 |
| South Australia |
51 |
41 |
80.4 |
1,770,800 |
2.88 |
| Australian Capital Territory |
20 |
12 |
60.0 |
431,500 |
4.63 |
| Northern Territory |
7 |
2 |
28.6 |
246,600 |
2.84 |
| Tasmania |
0 |
0 |
0.0 |
541,500 |
0 |
| Total |
6911 |
4414 |
63.9 |
25,694,400‡ |
26.90 |
Data obtained via Freedom of Information request #2370-02 to the Therapeutic Goods Administration, available at www.tga.gov.au/foi-disclosure-log
*Population data obtained from www.abs.gov.au/statistics/people/population/national-state-and-territory-population/dec-2020
†Per capita refers to number of approvals per 100,000 population
‡Includes other territories such as Jervis Bay Territory |
More than 190 different medicinal cannabis products can currently be accessed via TGA schemes,11 the most commonly prescribed being orally administered liquids (cannabis ‘oils’) with pharmaceutical grade cannabis plant material (‘flower’, ‘flos’ or ‘bud’) the second most common category (Figure 3). Other available products include capsules, sprays and wafers. Therapeutic actions of these medicinal cannabis products primarily arise from the cannabinoids Δ9-tetrahydrocannabinol (THC; the main intoxicating component of cannabis) and/or cannabidiol (CBD; a non-intoxicating component). Available products can be categorised as THC dominant or CBD dominant or can contain a mixture of THC and CBD (ratios of ~1:1 and ~1:10 are common). CBD-dominant products are in Schedule 4 of the Poisons Standard (>98% of total cannabinoid content must be CBD), while products with a significant amount of THC are in Schedule 8 and may require additional state-level permissions to prescribe. A recent rescheduling decision by the TGA means that CBD-dominant oral products containing <150 mg CBD and <1% THC may soon become available over the counter in Australian pharmacies.
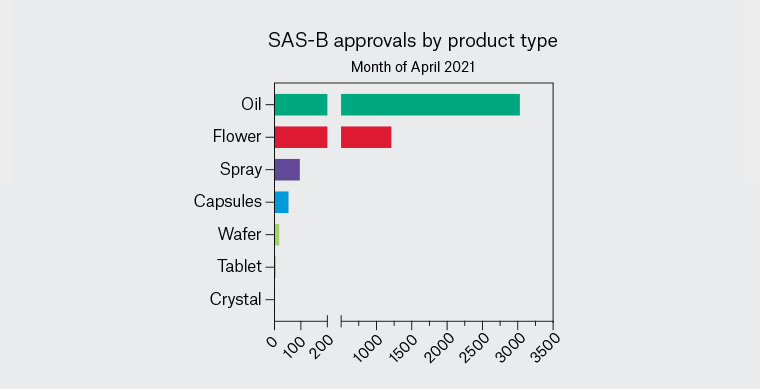
Figure 3. Types of medicinal cannabis products prescribed for chronic pain under Special Access Scheme Category B (SAS-B) in the month of April 2021. Data obtained through Freedom of Information request #2370 to the Therapeutic Goods Administration. Orally administered oils are the most frequently prescribed products, followed by cannabis plant material (‘flower’).
The aim of this article is to examine the use of THC and CBD in the treatment of chronic pain and summarise the evidence related to efficacy, side effects and optimal prescribing practices. For further information about the use of medicinal cannabis in palliative care settings, these authors recommend the article by Herbert and Hardy.12
The endocannabinoid system and pain
The analgesic effects of cannabinoids are well established in preclinical models of pain13 and arise primarily via interactions with the endocannabinoid system (ECS). The ECS is a ubiquitous system with multiple functions and comprises lipid signalling molecules called endocannabinoids (eg anandamide and 2-arachydonyl glycerol [2-AG]) that bind to specific cannabinoid receptors (CB1 and CB2 receptors) or other endocannabinoid-sensitive receptors (eg GPR18, GPR55, GPR119, TRPA1, TRPV1). Specialised enzymes regulate the synthesis, transport and degradation of endocannabinoids to maintain overall endocannabinoid tone.
The critical role for the ECS in pain and inflammation can be seen from the effects of experimental drugs that inhibit fatty acid amide hydrolase (FAAH; an enzyme that breaks down anandamide and other endocannabinoids). Inhibition of FAAH leads to elevated systemic endocannabinoid concentrations and analgesic effects in animal models of pain.13 In humans, a recent case study describes an elderly woman with a genetic polymorphism that reduces FAAH expression leading to elevated endocannabinoid concentrations and pain insensitivity.14 Reduced FAAH function is also associated with reduced need for postoperative analgesia in women undergoing breast cancer surgery.15
Phytocannabinoids
The cannabis plant contains >140 cannabinoids (known as phytocannabinoids), with THC and CBD the most abundant and the best characterised. Pharmacologically, THC acts a partial agonist at CB1 receptors, this being the primary mechanism behind its distinctive psychoactive effects, as well as analgesia and sedation. A synthetic structural variant of THC called nabilone also acts as a CB1 receptor agonist and is available by prescription in some countries for the treatment of chronic pain (for studies of efficacy, refer to Turcotte et al,16 Bestard et al17 and Berlach et al18). THC also acts as a partial agonist at CB2 receptors, which are widely expressed on immune cells and have a key role in inflammatory and immune processes. The role of CB2 receptors in mediating THC effects and in analgesia is not entirely clear; effects of CB2 receptor agonists on inflammation-induced pain are better described than their effects on nerve injury–related pain.13 Notably, a CB2 receptor–preferring agonist, lenabasum, is currently in late-stage clinical trials for the treatment of various inflammatory autoimmune conditions and fibrosis.19
CBD, in contrast to THC, does not directly activate CB1 receptors, and this explains its absence of intoxicating effects.20 CBD interacts, however, with a range of ECS-related receptors, and enzymes, to produce an array of anticonvulsant, anxiolytic, antipsychotic, anti-inflammatory and possible analgesic effects.21 While CBD appears analgesic in animal models, particularly those modelling neuropathic pain,13 there are minimal data related to analgesic effects in humans.
Evidence for the efficacy of cannabinoids in chronic pain
Overview of evidence
The evidence base for the efficacy of medicinal cannabis in treating chronic pain is complex and contentious. Numerous systematic reviews and meta-analyses have been conducted22,23 reaching both positive and negative conclusions. Although somewhat dated, a concise and useful review of outcomes is provided by the TGA’s Clinical guidance for the use of medicinal cannabis in the treatment of chronic non-cancer pain (December 2017).24 This analysis concluded that medicinal cannabis products were superior to placebo in producing a 30% reduction in pain scores and a 50% reduction in pain intensity ratings. However, the overall quality of evidence related to efficacy was low. A related review, published by Australian researchers in 2018, concluded that ‘evidence for the effectiveness of cannabinoids in chronic non-cancer pain is limited’.25 A more recent ‘review of reviews’22 concluded that the 57 systematic reviews of the literature over the past 20 years were ‘lacking in quality and cannot provide a basis for (clinical) decision making’. The array of studies reviewed in these systematic reviews involved a heterogeneous mix of cannabinoids, routes of administration, doses, pain conditions treated and outcome measures, with studies also differing on whether cannabinoids were used alone or adjunctively with other medications. It is striking that very few, if any, high-quality clinical trials have assessed the effects of the most commonly prescribed SAS-B products (ie orally administered cannabis oils) on chronic pain. Some key studies and outcomes are summarised in this article.
Inhaled cannabis
The most traditional route of cannabis consumption is inhalation of burned plant material, typically via bongs or joints. These modes are still widely used in the Australian community for self-medication with illicit cannabis,7 and herbal cannabis is now a popular prescription product that is inhaled via vaporisation (rather than smoking). Plant material currently accounts for nearly 30% of current SAS-B approvals (Figure 3),11 and a range of vaporisers have been approved by the TGA as medical devices for this purpose.26 The evidence relating to inhaled cannabis for chronic pain is varied. An early review of five clinical trials reported a >30% pain reduction in conditions such as diabetic and human immunodeficiency virus–related neuropathies.27 Other positive outcomes have been reported in trials of neuropathic pain.28–30 A large observational study involving thousands of Israeli patients documented reduced cancer-related pain and improved quality of life in patients using smoked cannabis across periods of several months.31
Nabiximols (THC/CBD oromucosal spray)
Nabiximols is an oromucosal spray containing a 1:1 ratio of THC/CBD that is currently listed on the Australian Register of Therapeutic Goods (ARTG). The main indication of nabiximols is spasticity in multiple sclerosis (MS), where it has well-demonstrated efficacy.32 Results relating to pain have been more mixed,23 including a marginal outcome in a large trial of nabiximols in cancer pain.33 Trials of nabiximols for pain associated with spinal cord injury,34 diabetes35 and chemotherapy36 have reached negative results; however, positive effects have been obtained in patients with brachial plexus avulsion37 and a mixed peripheral neuropathic pain.38 Analysis of a large German registry (n = 800 patients) indicated that 70% of patients reported a >50% improvement in pain after 12 weeks, with additional improvements in stress, depression, anxiety and overall wellbeing.39 Overall symptom relief/improvement scores favoured neuropathic pain over nociceptive pain.
Dronabinol (oral THC)
Dronabinol is synthetic THC in capsule form that is available on prescription in some countries, although not in Australia. Dronabinol has shown positive effects in patients with MS-related neuropathy40,41 but failed in a study of neuropathic pain related to spinal cord injury.42
Cannabidiol
There have been very few clinical trials exploring the analgesic effects of CBD in humans. While CBD is a component in nabiximols, the doses of CBD consumed in this product are very low (10–30 mg/day)and most likely inconsequential.43 In healthy volunteers, CBD had no clear analgesic effect in laboratory tests of pain thresholds and sensitivity,44 while a recent Australian clinical trial found that a single dose of adjunctive CBD (400 mg) was of no benefit in patients reporting to an emergency department with acute exacerbation of back pain.45 However, a study of 20 patients with chronic neuropathic pain reported superiority of 120 mg/day CBD over placebo.46 A recent observational study retrospectively assessed changes in quality of life in a subset of the first 400 New Zealand patients to receive prescription CBD (mostly 100 mg CBD/mL oil administered by dropper).47 In this study, patients with non-cancer pain (n = 53) reported significant improvements in pain-related quality of life, improved mobility and reduced anxiety and depression. Surveys of users in countries where cannabis products are more freely available (eg North America) suggest that CBD-dominant products tend to be more frequently consumed for anxiety and depression, while THC-dominant products are preferentially used for pain and sleep.48 Current SAS-B data indicate that almost a quarter of current approvals for chronic pain involve Schedule 4 CBD-dominant products, despite the minimal evidence available regarding efficacy (Figure 4).
![Figure 4. Other characteristics of Special Access Scheme Category B (SAS-B) approvals for chronic pain (month of April 2021) A. There were more approvals for men than women (0.2%25 did not specify gender); B. Approximately one-quarter of approvals were for cannabidiol-dominant (Schedule 4 [S4]) products, while the majority of approvals were for products containing substantial amounts of Δ9-tetrahydrocannabinol (Schedule 8 [S8]); C. Age breakdown of approvals showing very few approvals for patients under the age of 25 years.](/getattachment/AJGP/2021/October/Medicinal-cannabis/AJGP-10-2021-Clinical-Henderson-Medical-cannabis-Chronic-Pain-Fig-4.jpg.aspx?lang=en-AU)
Figure 4. Other characteristics of Special Access Scheme Category B (SAS-B) approvals for chronic pain (month of April 2021)
A. There were more approvals for men than women (0.2% did not specify gender); B. Approximately one-quarter of approvals were for cannabidiol-dominant (Schedule 4 [S4]) products, while the majority of approvals were for products containing substantial amounts of Δ9-tetrahydrocannabinol (Schedule 8 [S8]); C. Age breakdown of approvals showing very few approvals for patients under the age of 25 years.
Dosing and adverse effects
A major challenge with the use of cannabinoids is to weigh up potential harms for patients versus clinical benefits. For a detailed examination of cannabis-related adverse effects and of driving-related issues, these authors recommend the articles of Arnold49 and Arkell et al,50 respectively.
THC
THC has well-documented side effects including dizziness, appetite stimulation, drowsiness, altered mood, anxiety, and impaired cognition and attention. These effects vary by dose and route of administration, and rapid tolerance can occur to such effects. In clinical trials, treatment-emergent side effects of typical oral THC doses (approximately 5–20 mg) tend to be mild or moderate in severity and more prominent on the first day of dosing.51,52 Patients using nabiximols generally report few adverse reactions, other than mild increases in appetite and some dizziness, nausea, fatigue and dysgeusia. Inhaled cannabis will produce more immediate and pronounced feelings of intoxication than oral THC products.53 In clinical practice, doses of THC should be slowly titrated upwards from 2.5–5 mg/day to 10–20 mg/day to avoid feelings of acute intoxication and other side effects (eg anxiety). Regular monitoring of patients for adverse effects is recommended.54
There is little evidence of tolerance to the analgesic effects of cannabis-based medicines during extended use.32 Unlike with opioids, hyperalgesia to painful stimuli does not appear to occur with chronic use of cannabis,55 and analgesic effects can be retained, even when tolerance to psychotropic effects have developed.56 Heavy cannabis use in vulnerable individuals can increase the risk of psychosis and schizophrenia,24,57 and THC is contraindicated in individuals with a family history of mental health problems.58 Caution is also advised when prescribing THC to patients under the age of 25 years, and SAS-B prescribing data show that very few approvals for chronic pain involve patients in this age group (Figure 4). The possibility of drug-seeking behaviour should be considered in otherwise healthy patients requesting a cannabis prescription. Adequate risk stratification for substance use disorder is suggested prior to initiating therapy. Additional cautions with THC products include active mood or anxiety disorder, heavy alcohol or opiate use, and pregnancy and breastfeeding.25,54
Legal prohibitions regarding THC and driving are a significant barrier to patient use: patients with a legitimate medicinal cannabis prescription are not exempt from current drug-driving laws.59 Dosing THC by night reduces side-effect burden and minimises the complications caused by daytime intoxication given that impairment has a maximal duration of approximately 8–10 hours.60 Oral products are generally preferred to inhaled medicinal cannabis products because of issues related to respiratory health, although vaporised cannabis may allow more rapid relief for breakthrough pain, such as in cancer pain management.
CBD
CBD is well tolerated even at very high doses up to 6000 mg and has relatively benign side effects, the most common being diarrhoea.61 Other side effects such as somnolence, decreased appetite and fatigue are mainly evident when other medications are co-administered.62 Clinical benefits of CBD are best seen at doses of 300–1500 mg in epilepsy, anxiety, psychosis and addictions.43,63 However, such high doses are expensive, so many patients and prescribers dose CBD at approximately 60–200 mg/day.11,47 Prescribers should bear in mind the lack of evidence for efficacy of CBD at such low doses, although clinical trials using these dose ranges are underway.
CBD does not appear to impair driving and is not subject to current legal restrictions.59 Interactions between CBD and other prescription medications are possible given CBD inhibition of CYP450 enzymes.21 Interactions with the anticonvulsant clobazam are well documented in patients with epilepsy,64 and there are interactions with the commonly prescribed antidepressants citalopram and escitalopram that may increase their plasma concentrations.65 Upwards titration of CBD doses is therefore recommended as a precautionary principle, particularly in patients taking other medications.
Other considerations regarding clinical use
Cannabinoids and opiates
In addition to the effects on chronic pain itself, cannabinoids may reduce the requirement for patients to use conventional analgesics including opiates.66 CB1 receptors and mu-opioid receptors are colocalised in pain processing brain regions, functionally interact67 and are involved in placebo analgesia.68 A recent study of 97 patients with two-year stable opioid use for chronic pain found that a CBD-rich gel allowed 50% of patients reduce their opioid medications, with two eliminating their need for opioids entirely.69
Integrated approaches and benefits
The benefits of medicinal cannabis, in addition to potential pain reduction and opioid sparing, may include improved sleep, better quality of life and positive mood, all of which may contribute to improvements in chronic pain.70 This underscores the need to assess pain from a biopsychosocial perspective, including psychological, family, work and social influences. Management plans for chronic pain should include education, self-care strategies, behavioural management, multidisciplinary care, mindfulness, exercise and positive lifestyle. The use of cognitive behavioural therapy and stress management techniques is strongly recommended in addition to, or even instead of, pharmaceutical approaches or the use of medicinal cannabis.24,71
Withdrawal from products
Although medicinal cannabis is relatively safe when prescribed cautiously, patients with chronic pain who receive medicinal cannabis tend to be more likely to withdraw from clinical trials due to adverse effects than patients receiving placebo.24 Among people who use cannabis recreationally, abrupt discontinuation can produce a mild withdrawal syndrome characterised by sleep disturbances, depression and irritability, which typically peaks approximately two days following the last dose.72 Patients who have used THC products over several months or years are therefore advised to slowly taper off their use when withdrawing; inhaled cannabis can be replaced by oral products to facilitate dose titration during withdrawal.73 CBD has no addiction or dependence liability, and sudden abstinence does not lead to withdrawal.74 Indeed, CBD is currently of significant interest as a potential therapeutic option in the treatment of addictions including drug withdrawal.75
Conclusion
Medicinal cannabis is worthy of consideration in the management of chronic pain, and it is important that doctors are aware of the positives and negatives related to its use. The more commonly prescribed oral products (oils, sprays and capsules) are attractive since they can be delivered in a more controlled and socially acceptable manner than inhaled products, although they have a slower onset. While CBD products are attractive given better safety when driving or performing other safety-sensitive tasks, current supportive evidence for their efficacy is limited. Harm minimisation should always be front of mind in prescribing decisions, particularly with patients who are driving regularly or using heavy machinery. It must be recognised that the long-term effects of medicinal cannabis, potential drug–drug interactions and efficacy across different pain types remain only partly understood. The guiding principle of start low, go slow is crucial, with the aim to obtain clinical benefits at the lowest possible dose and to minimise risks and side effects.
A recent and authoritative systematic review, commissioned by the International Association for the Study of Pain, concluded that the current evidence ‘neither supports nor refutes claims of efficacy and safety for cannabinoids, cannabis, or cannabis-based medicines in the management of pain’ and that there is ‘the pressing need for studies to fill the research gap’,76 a conclusion supported by another recent systematic review.23 The Faculty of Pain Medicine of the Australian and New Zealand College of Anaesthetists concluded that until higher-quality evidence is available, currently available cannabinoid products should only be prescribed as part of a registered clinical trial.77
Despite the fact that current supportive evidence is of low overall quality, there are tens of thousands of patients with chronic pain being prescribed medicinal cannabis products in Australia, and hundreds of thousands using illicit cannabis products to self-medicate chronic pain.7,9 Many have legitimate lived experience of lasting pain reduction with cannabis that is not easily disregarded.7,78,79 There is clearly a disconnection between the pronouncements of specialist medical colleges and current prescribing and community use of cannabinoids in Australia. It is hoped that the results of the next generation of clinical trials of cannabinoid products and pain will help to resolve this tension.