Interview
Update on self-collected samples for HPV testing: Dr Lara Roeske Q&A
GP and cervical screening expert Dr Lara Roeske talks about the latest evidence on self-collected samples and its implications for under-screened patients.
 Dr Lara Roeske believes self-collection offers a powerful opportunity to engage under-screened and never-screened women in the cervical screening program.
Dr Lara Roeske believes self-collection offers a powerful opportunity to engage under-screened and never-screened women in the cervical screening program.
Dr Lara Roeske is a GP, liaison physician and co-author of the National Cervical Screening Program: Guidelines for the management of screen-detected abnormalities, screening in specific populations and investigation of abnormal vaginal bleeding.
She also works at the VCS Foundation. VCS Pathology is the only clinical laboratory in Australia accredited to process self-collected samples for human papillomavirus (HPV) testing as part of the National Cervical Screening Program.
New research published in the British Medical Journal, which includes work from the VCS Foundation, shows that self-collected samples for HPV testing provide similarly accurate results to clinician-collected samples.
Dr Roeske speaks with newsGP about this research and what it means in terms of being able to test women who have traditionally remained under-screened or never-screened.
What does the research show in terms of results for self-collected samples for HPV?
We now have evidence that HPV testing on self-collected vaginal samples has a sensitivity that is equivalent to that of a practitioner-collected sample for the detection of high-grade cervical lesions [CIN2+ and CIN3+ lesions] and is just slightly less specific at ruling out these lesions.
So this really is, in my view, giving GPs an absolute green light to offer this promising approach to women.
However, it is reserved for those patients who meet the eligibility criteria; that they are at least 30 years of age, and that they are under-screened or never-screened. For the purposes of our program here in Australia, that means four years or more since the last Pap test, and that they have refused the offer of a clinician-collected sample, which remains the gold standard.
These women are being identified in general practices and community health centres, so it’s not a mail-out kit, it’s not something where women front up to a pathology laboratory to get a kit – the test is managed by a GP in the practice. That’s very important.
Why can patients be offered self-collection for HPV testing in Australia?
We are able to offer self-collection because our new program is based on testing for high-risk or oncogenic HPV types. Pap smears or cytology-based screening is not amenable to vaginal self-testing. But now that we’re testing for the 14 known oncogenic HPV types that are associated with about 93% of cervical cancer in Australia, we can actually take samples from the vagina and we don’t need the sampling to occur from the cervix or the transformation zone.
The testing is done on a PCR [Polymerase Chain Reaction]-based platform, that is the validated platform we are currently using to test in Australia. And vaginal cells, which may contain the DNA or the RNA, the genetic material of the oncogenic HPV types, can be detected, if present, on the self-test.
What patient populations may benefit from self-collected sampling?
Although we have a well-organised screening program, and have had it for a number of decades here in Australia, 80% of cervical cancer in Australia occurs in women who are under-screened or never-screened.
We also know that screening participation can reduce or decline with increasing socioeconomic disadvantage. So there are particular groups of women that tend to be under or never-screened – women from Aboriginal and Torres Strait Islander populations, women who are rural or remote, women from linguistically and culturally diverse backgrounds, and women who may be experiencing socioeconomic disadvantage.
However, GPs will see many other groups of women who also, for a range of reasons, refuse a speculum exam.
We’re offering many women another option – such as older women who feel they no longer want to have screening because they’re no longer sexually active or their partner has died. Or for women who are victims of violence or sexual abuse, a speculum exam may be a barrier to them which we can now overcome with self-testing. Other important groups are transgender men, women who have sex with women.
We know that many of these women do see their GP for a whole lot of other reasons, and we would encourage GPs now to be more proactive about disrupting the traditional model of putting in a speculum and offering these women this opportunity to re-engage with the screening program.
It’s all about getting unscreened women participating in the program and hopefully, as a result, realising a reduction in cervical cancer incidence and mortality in this group of women.
What clinical information do GPs need to know about self-collected samples?
A dry-flocked swab is the swab that’s been validated for the Australian program. It’s known as a Copan 552C swab and that is the only swab, currently, that should be used.
There is a visual guide available online that can be used to assist women in understanding how to take the test. GPs should explain to women that they will be provided with a private area within the clinic to take the test, and that the swab needs to be inserted into the vagina. Women must also be reassured that it will not hurt.
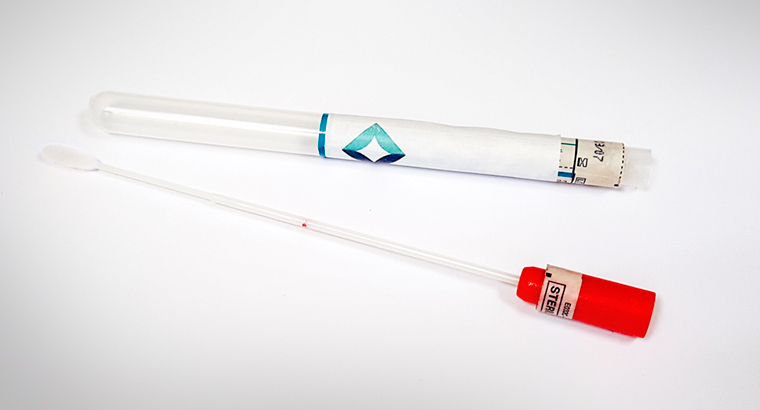 The Copan 552C dry-flocked swab is the only swab that has been validated for self-collection in the National Cervical Screening Program.
The Copan 552C dry-flocked swab is the only swab that has been validated for self-collection in the National Cervical Screening Program.
There is a clear mark at 8 cm on the stem of the swab which tells them the maximum amount it should be inserted. It should be rotated through one to three times, withdrawn and then returned to the tube and handed back to the GP or the nurse.
GPs should ensure they obtain a woman’s preferred contact details, and that they discuss follow-up around both negative and positive results.
Women with a negative result receive the same recommended screening interval of five years. When they come back at five years, they will be offered a clinician-collected test, but should they refuse that for any reason there is Medicare funding for a self-collect option at that return visit.
For women who have any oncogenic HPV detected, the self-test specifically identifies types 16 and 18, the most oncogenic HPV types. Women with a type 16 or a type 18 detected on a self-test should be referred for a colposcopic evaluation. GPs should note that colposcopic evaluation should occur in a timely way, preferably within eight weeks, because many of these women are under-screened, so we don’t know how long type 16 and/or 18 infection has been present in the cervix.
The women who have other oncogenic HPV types detected – other oncogenic HPV types not 16/18 – should return to the GP, and should be told they will get a call or a letter, whatever they prefer for being contacted, and will be asked to come back. At that visit, a practitioner-collected sample will need to be taken from the cervix. It is important to explain to women that we need this additional test to provide a cytology result which will inform the next step in management.
If any high-grade or glandular changes are seen on the cytology sample, then the woman should be referred for a colposcopic evaluation. Women who have a negative or low-grade changes on cytology should be asked to return in 12 months for a repeat HPV test.
GPs should ensure that the follow up options for positive and negative self-test results are explained to women at that consultation. We should also reassure women that this is a very accurate test.
For GPs who may be sending self-testing samples to VCS Pathology from remote and rural areas in Australia, the dry flocked swab has been validated for high temperatures, humidity, and also delays. So we’re confident that we are able, at VCS, to provide an accurate test result to GPs who may be sending samples to use from remote and rural areas in Australia,.
For GPs who would like to learn more, VCS Pathology also offers an RACGP-accredited QI&CPD 40 Category Point Clinical audit activity for HPV self-collection, available for the 2017–2019 triennium.
Is there anything else you want to add?
If GPs are targeting age-eligible, under-screened women who refuse a practitioner-collected sample, offering self-collection is a very powerful way to engage them in participation. There’s been some preliminary studies performed in Victoria and internationally that demonstrate that women do take up the offer – it is acceptable to them. Also, a very high percentage of women in these studies adhere with the follow-up.
That direct offer is very powerful.
This interview has been slightly edited for clarity.
cervical screening HPV human papillomavirus self-collection
newsGP weekly poll
What is your chief concern with role substitution?