News
Australian COVID-19 trials add convalescent plasma as a treatment
The plasma of recovered patients could be an additional therapy for people with the virus.
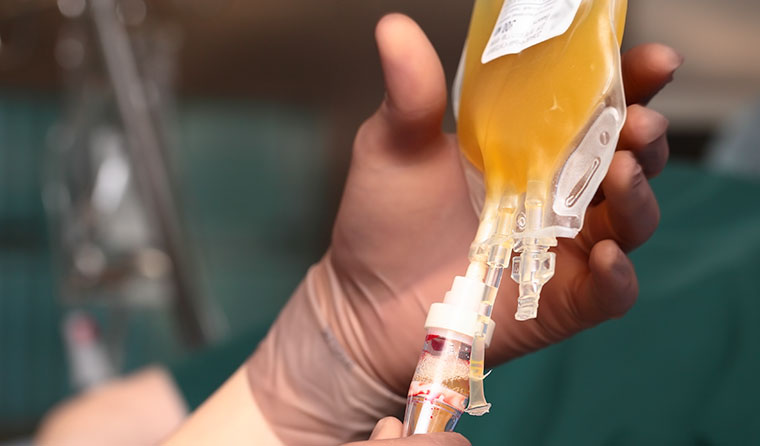 Convalescent plasma can be given to patients newly infected with coronavirus virus via a transfusion.
Convalescent plasma can be given to patients newly infected with coronavirus virus via a transfusion.
Patients who have recovered from COVID-19 have antibodies to parts of SARS-CoV-2 in their plasma.
Known as convalescent plasma, it can then be given to patients newly infected with the virus via a transfusion.
This may then result in more rapid control and clearance of SARS-CoV-2.
That is the basis for including convalescent plasma as a potential treatment option in two clinical trials on Australian patients hospitalised with COVID-19.
Those trials are the AustralaSian COVID-19 Trial (ASCOT), and the Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP), an Australian-led global study.
The first Australian patient was recruited to ASCOT at the Royal Melbourne Hospital on Tuesday, while REMAP-CAP will be recruiting intensive care unit (ICU) patients in coming days.
Royal Melbourne Hospital infectious diseases clinician Associate Professor Steven Tong is the principal investigator for ASCOT and a co-lead of clinical research at the Doherty Institute. He told newsGP convalescent plasma is ‘a promising avenue to explore’.
He says current treatments for COVID-19 include antivirals such as remdesivir, as well as anti-inflammatories such as dexamethasone.
Convalescent plasma, on the other hand, fits in with a different type of treatment known as antibody-directed therapy.
‘There may be other options within this kind of therapy,’ Associate Professor Tong said. ‘These include hyperimmune-globulin and monoclonal antibodies.
‘They all have a similar mechanism of action in that they contain antibodies that are directed against the virus.
‘It’s good to be offering a modality of treatment that is hopefully complementary to the standard treatment comprising dexamethasone and remdesivir that we’re providing at the moment.’
The ASCOT and REMAP-CAP trials are both complementary, but they explore COVID-19 at different stages of severity.
Professor Sharon Lewin, a leading infectious diseases specialist and Director of the Doherty Institute, told a press conference on Wednesday that ASCOT aims to capture patients with moderate to severe COVID-19 who are not yet in ICU, while REMAP-CAP looks at patients already in ICU.
Researchers remain hopeful about convalescent plasma due to previous findings relating to its role in other viruses.
‘Convalescent plasma has been used historically for the 1918 Spanish and the 2009 influenza pandemics, pneumococcal pneumonia, and also for previous coronaviruses – SARS and MERS,’ Associate Professor Tong said.
There have also been other studies into its safety.
‘Over 20,000 patients in the United States have safely received convalescent plasma for COVID-19,’ Associate Professor Tong said.

Associate Professor Steven Tong is excited about the inclusion of convalescent plasma as a treatment option in clinical trials for patients with COVID-19.
However, the efficacy of convalescent plasma as a specific treatment option for COVID-19 has not been evaluated on a significant scale.
‘It is disappointing that there haven’t been large trials conducted already,’ Associate Professor Tong said.
He says the only trial that has reported on its efficacy for COVID-19 was one done in China, which only had 103 patients and was terminated early, as the epidemic came under control.
Associate Professor Tong says while that study showed convalescent plasma did not make a statistically significant difference to COVID-19 outcomes, it was heading ‘in the right direction’ prior to its cessation.
‘We can’t really conclude much from it because of the size of the study,’ he said.
Associate Professor Tong says there are trials in the UK that have included convalescent plasma as one of their treatment arms, but there will not be a ‘definitive answer’ on its efficacy as a supplementary treatment option until further research is completed.
He hopes ASCOT – which is conducting a randomised, controlled study into the use of convalescent plasma along with standard care, versus standard therapy alone – will add further data to this field.
‘It’s not going to be a silver bullet, but it will hopefully add to what we’re already doing,’ he said.
More plasma is needed from recovered patients in order to further evaluate the role of convalescent plasma on patients currently infected with COVID-19.
Associate Professor Tong believes clinicians should encourage patients who have recovered to consider donating their blood and plasma to the Australian Red Cross Lifeblood.
‘That could actually be something that helps someone else who gets COVID-19 later on,’ he said.
Dr James Daly, Medical Director of Pathology Services at Lifeblood, echoes that sentiment.
‘Donating plasma is a simple, powerful act that could help a patient struggling to fight the disease,’ he said.
‘It’s a real opportunity for people who have battled COVID-19 to become part of a potential solution.’
Log in below to join the conversation.
convalescent plasma coronavirus COVID-19
newsGP weekly poll
Health practitioners found guilty of sexual misconduct will soon have the finding permanently recorded on their public register record. Do you support this change?