News
Call on COVID vaccine for under-fives likely ‘in coming weeks’
With mRNA vaccines for children aged under-five now approved in US, authorities are actively considering a similar move in Australia.
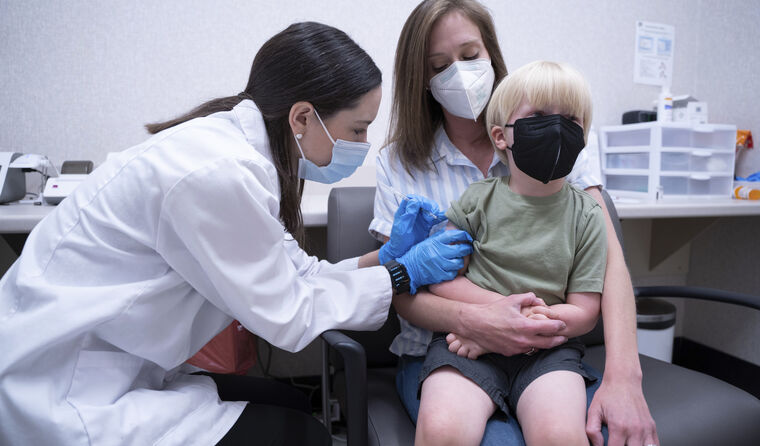 A three-year-old child is among the early recipients of the newly approved COVID-19 vaccine in the US.
A three-year-old child is among the early recipients of the newly approved COVID-19 vaccine in the US.
A decision on using the Moderna COVID-19 vaccine for children under the age of five is likely in the next few weeks, Federal Minister for Health and Aged Care Mark Butler said on Tuesday.
It follows the approval by the US Food and Drug Administration (FDA) for the emergency use of two mRNA vaccines – Moderna and Pfizer – for children aged older than six months.
The move was first set out by the US Advisory Committee on Immunization Practices, and subsequently endorsed by the US Centers for Disease Control and Prevention (CDC).
The FDA stated that the Moderna vaccine for children aged six months to 5-years-old is recommended as two 25 microgram doses administered at least a month apart. There will also be a third dose for high-risk children, the organisation said.
‘The vaccine is also authorised to provide a third primary series dose at least one month following the second dose for individuals in this age group who have been determined to have certain kinds of immunocompromise,’ it said.
The CDC stated on Saturday that vaccination can take place with immediate effect, opening up the vaccine program to almost the entire US population.
‘All children, including children who have already had COVID-19, should get vaccinated,’ they stated.
In an ABC interview on Tuesday morning, Minister Butler noted that Australia has tended to follow the same pattern as the United States with vaccine approvals.
‘You’ve generally seen a sequence whereby the American authorities will consider vaccines first of all, then the Europeans, and then countries like Australia,’ he said.
‘Now the TGA [Therapeutic Goods Administration] … has an application from Moderna which they’re currently going through. If that is approved, then that will go, as I said, to our advisory group on vaccinations.
‘I don’t control that timeframe. I think it’s appropriate that those authorities feel that they are able to conduct their work, their legislative work, in a timeframe that’s proper.
‘And I’d expect that to happen over coming weeks.’
The TGA confirmed receipt of an application from Moderna last month, which it said it would begin evaluating immediately.
Pfizer has not lodged an application for COVID-19 approval in the youngest age group in Australia so far.
Its vaccine has been approved for emergency use in children aged five and older, while the Moderna vaccine can currently be administered to children aged six and over according to TGA guidelines.
On Tuesday, Nine Newspapers also reported about the possibility of an mRNA vaccine that simultaneously targets new SAR-CoV-2 variants and other respiratory illnesses being made on Australia territory.
The development of a vaccine targeting flu, COVID and RSV has been underway at Moderna since last year.
Moderna’s Chief Medical Officer Dr Paul Burton told media this week that such a vaccine is ‘just around the corner’, and that he hopes to secure approval by late 2023 or early 2024.
The company also suggested that if such a vaccine is approved, it could be made at its new mRNA manufacturing facility in Victoria, which is expected to begin operating in 2024.
Log in below to join the conversation.
ATAGI child vaccines COVID-19 vaccination TGA
newsGP weekly poll
How often do patients ask you about weight-loss medications such as semaglutide or tirzepatide?