Column
Does new evidence for paracetamol preventing febrile seizures stack up?
Dr Casey Parker reviews a recent paper from the paediatric literature that has ‘created a small stir’.
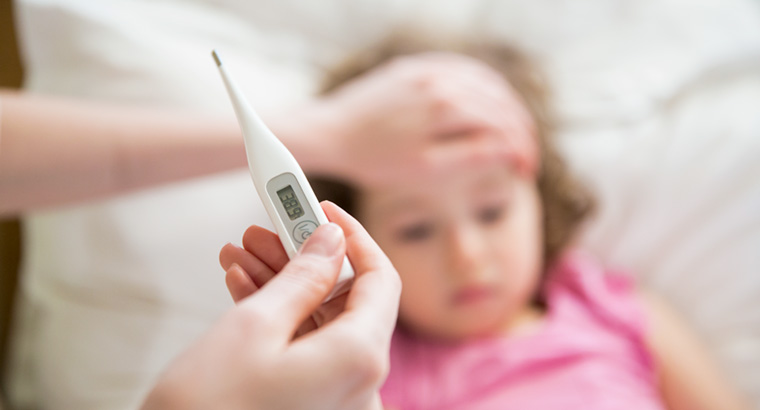 Is paracetamol useful in treating febrile seizures?
Is paracetamol useful in treating febrile seizures?
Ever since I trained, we have managed simple febrile seizures with no active intervention other than symptom relief and watchfulness.
The paediatric party-line, based on some data, is that using antipyretic medications like paracetamol will not change the prognosis or rate of recurrence of subsequent seizures.
However, a new paper in Pediatrics, Acetaminophen and febrile seizure recurrences during the same fever episode, has come to a different conclusion, finding rectally administered paracetamol is effective at preventing recurrence of febrile seizures.
I thought I would run the evidence-based rake over this new data and try to decide how it fits in with our current practice, and whether we should change our advice based on this evidence.
Let’s start with a PICO (Population, Intervention, Control, Outcome) model to look at the data.
Population
The study looked at children with simple febrile seizures at a single Japanese paediatric emergency department.
The risk with this type of small, single location trial is that it may not be generalisable to the wider population. The local genetics, microbiology and environment may be quirky.
Febrile seizure are generally defined as a seizure accompanied by fever (body temperature ≥38°C), without central nervous system infection that occurs in infants and children six to 60 months (five years) of age.
The study’s exclusion criteria included patients who had:
- already experienced two or more febrile seizures during the current fever episode
- seizures lasting >15 minutes
- epilepsy, chromosomal abnormalities, inborn errors of metabolism, brain tumour, intracranial haemorrhage, hydrocephalus, or a history of intracranial surgery
- been administered diazepam suppository to prevent febrile seizures and patients whose parents requested the use of diazepam suppository
- taken antihistamines (may decrease seizure threshold)
- diarrheal illness (may have gastro-associated seizure).
Here is how enrolment played out:
- 794 screened for febrile seizures
- 279 excluded
- 17 refused to participate
Of those remaining, 438 were randomly assigned; 60 had diarrhoea and were thus not included in the treatment or had no treatment protocol.
Of the 438, 229 received rectal acetaminophen (paracetamol) and 209 received no antipyretics.
A few things to note
Nearly a quarter of the children screened received per rectum diazepam to prevent a seizure. That seems like a very high number; it is basically the same as the number of children who had had a prior febrile seizure on a previous episode of febrile illness.
So, my guess is that it is common practice for Japanese children to be prescribed per rectum diazepam for this scenario. This differs from Australian practice.
This doesn’t really change the outcomes, as these children were excluded. But it does make me wonder if the numbers could have been diluted/skewed by selecting a subset of children who proportionally will be ‘first timers’ and thus may include more ‘undiagnosed epileptic’ children.
Otherwise, the two groups looked similar at baseline.
Intervention/Control
The two groups were randomly allocated to receive either:
- rectal acetaminophen (paracetamol) suppository at a dose of 10 mg/kg immediately and then every six hours for 24 hours if they remained febrile (T >38°C)
- no intervention – there was no placebo suppository used in this trial; parents were instructed to avoid any antipyretics for the first 24 hours.
There are a few issues with this trial design:
- This is a no-placebo trial, which is problematic. Giving a medication rectally is quite a significant thing to do to a small child, and doing it four times a day more so. There is certainly a risk of bias here. It is not implausible that this may have some effect on seizures via the vagal-stimulation pathway.
- Ten mg/kg is a low dose; most emergency departments would go with 15 mg/kg and some load with 20 mg/kg. So it is hard to convert this intervention into our practice, though a low dose would tend to favour the null hypothesis.
- There is an ethical question related to instructing parents to stay away from antipyretics in the control group. These children are unwell with fevers and are often miserable. Is it ethical to withhold symptomatic relief in the context of a trial? Sure, it makes the science clearer, but at what cost?
 The new study looked at children with simple febrile seizures at a single Japanese paediatric emergency department.
Outcome
The new study looked at children with simple febrile seizures at a single Japanese paediatric emergency department.
Outcome
The primary outcome was seizure recurrence rate in the same acute illness (all events occurred in the first 24 hours). The seizures were reported by the parents, so a few may have been incorrectly identified events (eg rigors).
In the first 24 hours, 16% of all included children had a subsequent seizure during the same episode of illness.
Broken down, that was 9.1% in the per rectum paracetamol group and 23.5% in the ‘no intervention’ arm.
This yielded a very impressive p-value of <0.001.
If you believe the numbers, then I think that as a parent I would consider this a clinically significant effect, too.
A 14.4% absolute risk reduction gives a number needed to treat of about seven.
The calculated odds ratio was 5.6 (CI = 2.3–13.3). This seems like a potent intervention.
Discussion
This is an interesting study that asks an important clinical question. The data regarding the treatment of febrile seizures is not really that robust, with only a few small trials looking at acute seizure recurrence.
To summarise my PICO
The study used sensible inclusion and exclusion criteria. However, the large group that was excluded (rectal diazepam pre-treatment) makes me wonder if the baseline population is truly reflecting the ‘natural population’ in the community.
The intervention design was potentially problematic – low dose, no placebo and slightly ethically troubling. I think a placebo suppository would have been useful to eliminate doubts about the effect of the intervention providing manual stimulation and unintended therapeutic action.
It is possible that the parents’ reporting inflated the rate of seizures, but we will never know.
My big concern with this study is the 16% overall recurrence rate – this is very high. When I asked a few experienced doctors, that rate seemed to be around five times what we see in practice.
The authors also cited a previous
European Journal of Pediatrics paper on this question. In this smaller trial (50 in each arm), the recurrence rates were only 7–8%, meaning in that cohort the placebo group had lower rates of recurrence than in the treatment group in this Japanese data.
This makes me wonder whether we may be dealing with a population in this Japanese centre that either has some predilection to febrile seizures, or whether the population was artificially selected (maybe by excluding the diazepam users) to create a group of children who had a very high baseline risk for recurrent seizures.
The children who had the diarrheal illness and were subsequently excluded from the study went on to have an astronomically high seizure recurrence rate of 35%. This is potentially due to some local microbiological nasty that secretes an endotoxin that jiggles young brains.
Given this common occurrence, it is entirely possible that some of the study population was ‘contaminated,’ meaning they had the same microbe in their systems but had not yet developed symptoms.
This is just a postulation, but it would explain the high numbers at baseline to some extent. Unfortunately, the kids were not followed to see who went on to be like Don Bradman and get a heap of runs.
Bayesian bottom line
Prior to seeing this data, I/we did not believe that there was a significant effect from the use of antipyretics in the prevention of subsequent seizures in children with simple febrile seizures.
This is a small trial that has some design flaws and potentially does not represent our natural population.
Despite a significant result, I do not believe this data can change the probability for efficacy enough to make this a ‘practice changer’.
So, as you were.
Final thoughts
Simple febrile seizures (and even recurrent febrile seizures) are a benign condition. However, they are scary and not something any parent wants to endure.
As doctor–scientists, we do need to decide which interventions are useful. The recurrence of a seizure in this trial was counted as a fail – a bad outcome.
But when you think about it, if the child comes to no real harm and we can educate and reassure parents, is it really a harm? What exactly are we preventing with an invasive yet safe therapy such as rectal acetaminophen?
FYI: I cannot recall the last time I ordered/gave per rectum medication to an awake/well child. Can we do the trial with orals next time?
clinical trials evidence-based medicine febrile seizures fevers paediatrics
newsGP weekly poll
Health practitioners found guilty of sexual misconduct will soon have the finding permanently recorded on their public register record. Do you support this change?