News
Novavax approval: what it means for GPs
The TGA’s Professor John Skerritt hopes the availability of the new vaccine will push the vaccination rate in Australia even higher.
 The Novavax vaccine is the fourth to be approved in Australia. (Image: AAP)
The Novavax vaccine is the fourth to be approved in Australia. (Image: AAP)
The Therapeutic Goods Administration (TGA) has given provisional approval to the protein-based Novavax COVID-19 vaccine, with hopes it will further boost Australia’s vaccination coverage.
The approval covers the administration of Novavax, which goes under the brand name NUVAXOVID, as a primary course vaccination for people aged 18 and older.
GPs are expected to be involved in the rollout of the vaccine, which is the fourth to be approved for use in Australia.
It had originally been due for approval last year but was delayed.
The TGA announcement follows approvals in India, the European Union, Indonesia and the Philippines.
The vaccine has not been given the green light to be used as a booster in Australia, nor has it been approved for use in children.
The TGA’s national manager Professor John Skerritt said on Thursday that data is still being considered.
‘I know there is interest in the potential for Novavax being used as a booster or even as an adolescent paediatric dose, but we are talking to them and we have been giving an undertaking, as we have with all vaccines, that as soon as we get the data we will review it as an absolute top priority,’ he said.
In a press conference announcing the drug’s approval the Federal Health Minister Greg Hunt confirmed general practices will be involved in the rollout of the vaccine.
‘In relation to Novavax, COVID Shield is working with general practices, pharmacies and state clinics and Commonwealth and they will take orders,’ he told reporters on Thursday morning.
‘We do have significant quantities that will be coming and made available and we want to lift as many of those people who haven’t yet been vaccinated into the vaccinated groups.’
Professor Skerritt also said he hopes the approval will raise the vaccination rate even higher.
Many general practices have reported patients indicating an intent to delay vaccination until Novavax was approved, which will be the first protein-based COVID-19 vaccine available in Australia.
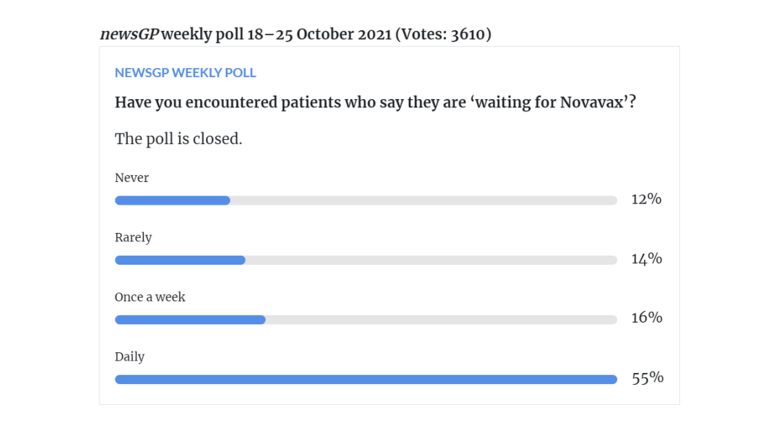
In a poll conducted by newsGP in October last year, more than half of respondents reported daily inquiries from patients saying they were ‘waiting for Novavax’.
Professor Skerritt said it is hard to quantify how many more people will come forward for their primary vaccination course when the Novavax vaccine is available but said the TGA has fielded many inquiries.
‘I don’t know whether it is 50,000 or 100,000 or a million or whatever individuals [who will come forward for vaccination], I don’t think anybody knows but there are some individuals, and this just gives them further choice,’ he said.
The arrival of Novavax supplies could also allow Australia to provide vaccines to countries in the Asia-Pacific region where the vaccination rate is still lagging, he said.
The Australian Technical Advisory Group on Immunisation (ATAGI) will need to confirm its own guidelines for the vaccine before it is used in Australia.
The first shipment of the vaccine is due to arrive next month, with the initial supply coming from the Serum Institute of India, the world’s largest vaccine manufacturer by volume of doses. On arrival to Australia, it will be tested locally.
A spokesperson for Sydney-based company Biocelect, which acts as the sponsor for the Novavax vaccine in Australia and New Zealand, said the exact quantity of doses that will arrive in the first shipment is yet to be confirmed.
‘We will work diligently with regulatory authorities and our partners to expedite the final steps to get our vaccine to the public,’ they said.
‘We will have a better sense of the number of doses available for Australia in the coming weeks. We are producing at a capacity of 150 million doses per month and we expect our global supply chain to achieve capacity in excess of two billion doses in 2022.’
The vaccine will be packaged in a vial including 10 doses in ready-to-use liquid form. The primary vaccination course consists of two 0.5ml doses administered three weeks apart.
Storage of the vaccine is from 2–8°C, which means existing storage and supply channels can be used. Its shelf life is stated as six months.
In January last year, the Department of Health announced an agreement with the US-based biotechnology company for the supply of 51 million doses of the vaccine, with an option for 10 million more if required.
A clinical trial in the United States and Mexico with 29,949 participants, which took place from December 2020 to February 2021, achieved 90.4% efficacy in preventing COVID-19 infection.
A smaller trial in the UK showed similar results.
Novavax vaccine’s phase 1/2 study took place in Australia. Infectious diseases physician and microbiologist Associate Professor Paul Griffin of the University of Queensland was the principal investigator in that trial. He also believes the approval of Novavax is likely to improve Australia’s already high vaccine uptake.
‘For a variety of reasons, it seems some have remained reluctant to receive these vaccines thus far so an additional option, based on what is considered perhaps a more traditional platform, is likely to increase our vaccination rate even further,’ he said.
The findings of both the US/Mexico and UK trials have now been published in the New England Journal of Medicine.
The most common adverse reactions reported were headache, nausea, myalgia, arthralgia, as well as tenderness around the injection site and fatigue.
The company has also begun work on an update to its vaccine to specifically target the Omicron variant.
The Biocelect spokesperson said it is too early to know whether a switch to a variant strain vaccine will be the right approach.
In its own announcement of the TGA’s approval, Novavax said there is also currently a phase 1/2 trial for a combination seasonal flu and COVID-19 vaccine taking place in Australia.
Log in below to join the conversation.
COVID-19 general practice Novavax NUVAXOVID vaccine vaccine rollout
newsGP weekly poll
Within general practice, do you think there are barriers to providing flu vaccinations? If so, what are they?