News
Oral antivirals access opens to more Aboriginal Australians
The move will come in with PBS changes from 1 November, which also include a treatment for a rare genetic disease.
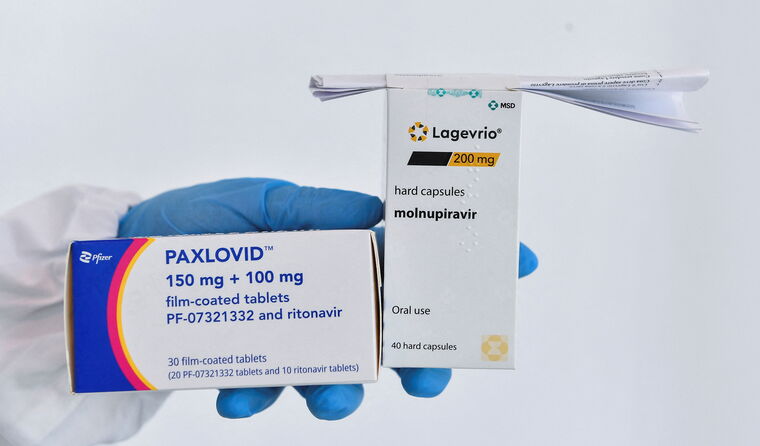 Access for the COVID-19 oral antivirals will be further widened from next month.
Access for the COVID-19 oral antivirals will be further widened from next month.
Aboriginal and Torres Strait Islander people aged over 30 will only require one risk factor to be eligible for COVID-19 oral antiviral treatments from the beginning of next month.
Previously, two risk factors were required among the cohort for access to molnupiravir (sold as Lagevrio) and nirmatrelvir plus ritonavir (sold as Paxlovid), both of which were listed on the PBS earlier this year.
Among other changes to the PBS from 1 November, a range of new medications will add options for a common skin cancer, lung cancer patients, and those diagnosed with X-linked hypophosphataemia (XLH), a rare genetic disease.
The listings were confirmed by the Department of Health and Aged Care (DoH) in an announcement made on Monday (24 October).
Cemiplimab, sold as Libtayo, was included in the list, and will be available for the treatment of locally advanced or metastatic cutaneous squamous cell carcinoma, one of the most common skin cancers.
‘This is the first PBS-subsidised immunotherapy available to Australians with this cancer,’ the DoH stated, as well as estimating that an average of 1000 patients per year could benefit.
Meanwhile, burosumab (sold as Crysvita), the treatment for XLH, was singled out by Federal Health and Aged Care Minister Mark Butler at a press conference in Adelaide on Monday.
‘This is a rare condition that affects several hundred Australians at any one time,’ he said. ‘And this listing is the first new substantial therapy for this condition for decades.
‘Australia … will be one of, if not the first, country on the planet to have listed this life-changing, lifesaving medicine, not just for children, but for adults with this condition as well.’
Professor Peter Ebeling, an endocrinologist and Head of the Department of Medicine at Monash Health, described the listing as ‘a major milestone’ in the treatment of the disease.
‘For the first time, we are able to provide XLH patients a treatment that addresses the underlying biochemical cause of the disease, rather than just a “band-aid” solution,’ he said.
‘Now, we can stop the loss of phosphate that causes the multiple health problems seen in XLH rather than trying to close the gate once the horse has bolted.’
Two further medications will be listed on the PBS as treatments for non-small cell lung cancer (NSCLC), which could help a combined total of around 520 people each year, the DoH estimates.
Atezolizumab, sold as Tecentriq, is being expanded as an adjuvant treatment for some patients with Stage II to IIIA NSCLC who have had surgery and chemotherapy.
Tepotinib, sold as Tepmetko, is also being listed for the treatment of locally advanced and metastatic NSCLC with a particular type of gene alteration (METex14sk).
Log in below to join the conversation.
Aboriginal and Torres Strait Islanders oral antivirals PBS
newsGP weekly poll
As a GP, do you use any resources or visit a healthcare professional to support your own mental health and wellbeing?