News
People experiencing psoriasis and asthma receive PBS relief
Patients with urea cycle disorder will also benefit from the new round of listings.
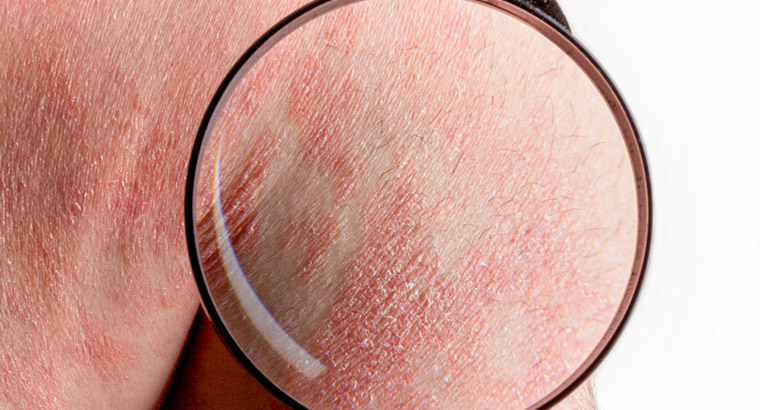 People experiencing psoriasis and asthma have gained improved access to some drugs under the PBS.
People experiencing psoriasis and asthma have gained improved access to some drugs under the PBS.
Close to 9300 patients with moderate-to-severe plaque psoriasis are now able to access risankizumab [sold as Skyrizi] through the Pharmaceutical Benefits Scheme (PBS), having previously being required to pay up to $32,000 per year for treatment.
The listing, which will see patients pay a maximum of $40.30 per script ($6.50 for concession card holders), follows a July recommendation from the Pharmaceutical Benefits Advisory Committee (PBAC) on a ‘cost-minimisation basis’.
Expanded access criteria for medicines benralizumab and mepolizumab (sold as Fasenra and Nucala, respectively) will allow an additional 1000 patients with severe eosinophilic asthma to access the medications through the PBS, saving them up to $23,000 per year.
The PBAC recommended several changes to PBS restrictions for biologic medicines for severe asthma in May, based on the outcomes of the December 2018 asthma stakeholder meeting.
The amended criteria:
- Initial treatment
- Amendment of the eosinophil cut-off from ≥ 300 cells per µL to 150 cells per µL for patients on oral corticosteroids
- Removal of the forced expiratory volume (FEV1) ≤ 80% predicated normal
- A 32-week initial treatment period across all three medicines
- Continuing treatment
- Continuation of therapy where Asthma Control Questionnaire (ACQ) is no greater than 0.5 higher than baseline where oral corticosteroid (OCS) dose has been reduced
- Switching
- A new ‘initial 2’ restriction for patients switching therapy to remove the current requirement for a six-month break between treatments
- Re-trialling and cycling
- Re-trial of the same biologic after previous failure following a 12-month break (increased from the current six-month break)
The PBAC also recommended removing the need for radioallergosorbent (RAST) testing for omalizumab, and replacing it with ‘past or present evidence of atopy, documented by skin-prick testing or an in vitro measure of specific IgE’, but the Government made no mention of this
in its release.
The final new listing will see around 125 Australians with urea cycle disorder (UCD) save up to $19,000 on scripts for sodium phenylbutyrate (sold as Pheburane).
The PBAC recommended the Authority Required listing of a sugar-coated granule formulation in July, after
initially rejecting sponsor Orpharma’s submission on primarily economic grounds.
asthma PBAC PBS Pharmaceutical Benefits Advisory Committee Pharmaceutical Benefits Scheme psoriasis Urea cycle disorder
newsGP weekly poll
Which of the following areas are you more likely to discuss during a routine consultation?