News
Questions raised over respirators used in Australian hospitals
Victorian and NSW public hospitals are using a batch of respirators that are at risk of tearing and may not meet Australian manufacturing standards.
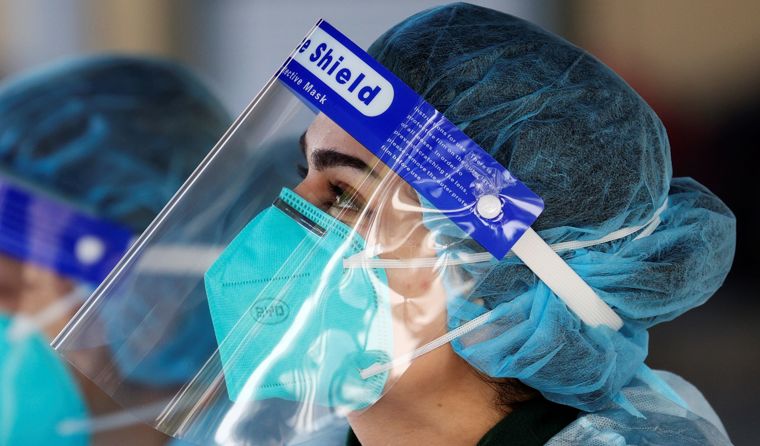 Victorian health authorities this week cautioned that the BYD respirators are at risk of tearing if adjusted, following a NSW Health alert issued on 20 August.
Victorian health authorities this week cautioned that the BYD respirators are at risk of tearing if adjusted, following a NSW Health alert issued on 20 August.
Experts have expressed concern over the use of the Chinese-made BYD DE2322 N95 face masks in some Australian hospitals, which were manufactured prior to receiving certification from the US regulator responsible for N95 compliance.
The masks did not gain National Institute for Occupational Safety and Health (NIOSH) certification as an N95 respirator until 7 June; however, many of the respirators currently in use across Victoria and New South Wales come from a batch manufactured on 5 May.
BYD – a Chinese electric-vehicle manufacturer that began making masks in March this year – was forced to refund half of the state of California’s down payment for its US$1 billion mask-supply deal until it could gain NIOSH certification, according to the San Francisco Chronicle.
Victorian health authorities this week cautioned that the BYD respirators are at risk of tearing if adjusted, following a NSW Health alert issued on 20 August seen by newsGP.
A Department of Health and Human Services (DHHS) spokesperson told newsGP the department is ‘closely monitoring’ advice about the BYD respirators.
‘[B]ased on the current information, we’re confident that they are keeping users protected,’ the spokesperson said.
In a public alert, Safer Care Victoria sought and received assurances from BYD that there were no changes to the respirator design, raw materials or construction between lodging the application and receiving NIOSH approvals.
But leading occupational hygienist Kate Cole has questioned whether these assurances are adequate, raising the issue with the Therapeutic Goods Administration (TGA) and Safer Care Victoria.
‘It is apparent that the BYD DE2322 masks, prior to receiving NIOSH approval, were not able to be used in the USA. Yet, concerningly, such products have made their way into the Victorian healthcare system,’ she told newsGP.
The non-NIOSH-approved BYD DE2322 is not listed on the Australian Register of Therapeutic Goods (ARTG) as an N95 respirator – the US standard similar to Australia’s P2 – but as a Chinese-standard KN95.
However, the packaging seen by newsGP claims the mask is an N95 and meets the more stringent Australian P2 standard.
‘BYD DE2322 masks manufactured prior to 9 June have been brought in under the ARTG as a KN95, not an N95,’ Ms Cole said.
‘None of the test results provided by the manufacturer meet that intended purpose. They claim it’s an [Australian-standard] P2, but we don’t have evidence for that.’
Wing You, General Manager of BYD Australia, told newsGP the respirator is ‘fully compliant with Australian standards’ and the batch is ‘definitely’ safe.
‘These masks were manufactured in May. They didn’t get approval from NIOSH, but they are the same model,’ he said.
‘The reason for the delay is that when we submitted some documents, there were some mistakes. We had to withdraw and resubmit. But the product, the raw material, the mask design, that had no changes.
‘When we sent this mask to Australia, we didn’t promise that it was NIOSH-approved; just that it was the same model.’
Mr You provided newsGP with the declaration his company gave to Victorian and NSW health authorities, which states there are no changes in ‘design, raw materials or construction’ of the respirators between applying for and receiving NIOSH approval, and also since testing the masks.
The declaration attributes the delay in NIOSH approval to documentation mistakes.
Mr You also provided the passing results of these respirators from NIOSH testing in April. He said the testing is ‘equal to’ Australian standards.
In response to concerns of tearing, Mr You said users should not adjust the straps themselves, that small tears around the staples ‘would not change the protective level’, and that other brands of respirators use the same staple to fix the strap.
According to a company statement from May, ‘BYD is manufacturing a high-quality product that has already passed NIOSH exhale, inhale and N95 NaCl tests’.
But Ms Cole points out that P2-standard testing includes ‘additional tests such as total inward leakage aimed at ensuring the mask fits snugly, which American-standard N95s do not have to pass’.
The Wall Street Journal reported in May that the initial NIOSH rejection came after an on-site assessment of BYD factories in China yielded a rating of ‘not acceptable’ and that a review of documentation around design, manufacturing and quality inspection was ‘concerning’.
The concerns come as Australia’s TGA moves to pull hundreds of different varieties of face mask from the register of therapeutic goods over safety concerns.
Hundreds of different manufacturers entered into mask-making during the early phase of the COVID-19 pandemic, as nations around the world competed to get supplies of scarce personal protective equipment (PPE).
Citing the need for urgency, Australia’s TGA lifted the requirement that all masks be tested prior to being registered on the ARTG.
newsGP is not suggesting BYD has done anything wrong in making the masks available in Australia.
A TGA spokesperson told newsGP all face masks on the ARTG are subject to the review, and BYD ‘provided test reports to the TGA to support the safety and performance of the devices’. TGA Laboratories and independent testing laboratories are now seeking to validate these results, the spokesperson said.
‘Test results will be published on the TGA website and regulatory action will be taken if there are any safety concerns identified,’ the spokesperson said.
‘The TGA is working closely with the state and territory health departments to keep them informed on the progress of all assessments.’
In June, a coalition of workplace health and safety organisations called for a national register of approved respirators, given the confusion regarding which were safe to use.
Australian Institute of Occupational Hygienists president Andrew Orfanos told newsGP a register of approved respirators would make it much easier for procurers to purchase safety equipment with confidence.
‘Unfortunately, because of the demand for PPE and with so many new brands, the TGA had to expedite the process because they didn’t have the time or resources to test all the masks,’ he said.
‘They provided a certificate saying this can be sold provided they are fit for purpose. It is effectively self-regulation.
‘But the problem is, people are taking that certificate and saying, “Look, it’s TGA-certified”. That was not the case – it was a way of expediting the issue.’
The coalition’s calls for change came at the launch of a guide to buying P2 or equivalent respirators.
The guide may help GPs with decisions over mask purchases, given many GPs have been forced to supplement Commonwealth-supplied PPE with commercial purchases of masks.
Log in below to join the conversation.
face masks N95 New South Wales PPE Victoria