News
TGA to provide further clarity on vaccine advertising guidelines
The move comes after a request from RACGP President Dr Karen Price.
 Practices are only able to provide factual information that would assist the public in obtaining a COVID-19 vaccine. (Image: AAP)
Practices are only able to provide factual information that would assist the public in obtaining a COVID-19 vaccine. (Image: AAP)
GPs have been left in limbo after the Therapeutic Goods Administration (TGA) issued a warning last week against promoting the use or supply of the coronavirus vaccine, amid concerns it risks breaching Australia’s ban on advertising prescription medications.
With phase 1b rolling out in general practices in less than a week, RACGP President Dr Karen Price has moved to help GPs gain more clarity.
‘I have asked [TGA boss] Professor John Skerritt directly to clarify the TGA’s advice with regards to how GPs talk about the COVID vaccine, and he has assured me that the TGA will be issuing formal guidance for general practices,’ she told newsGP.
The TGA issued guidelines on advertising COVID-19 vaccines to the Australian public on its website last week.
It states that advertisers ‘must not use self-developed advertising’ about COVID-19 vaccines, and instead opt for materials produced by federal, state or territory governments, in the same way they are used to promote influenza and other vaccines.
‘Government produced materials may be used in all communication channels including on websites, as flyers, or as posters as well as in the windows and on the walls at vaccine providers, clinics, pharmacies, and other businesses,’ the TGA website states.
‘Businesses with an interest in disseminating information about the availability of the vaccine may also use the materials in their newsletters, social media and emails.’
Practices are also able to provide factual information to assist the public in obtaining the vaccine, such as the location of vaccination service, times vaccines are administered or opening hours of the service provider, as well as whether there is a need for an appointment to receive the vaccination and how to make one.
While using the promotional material, practices are advised to be careful not to add any of the following:
- the tradename and/or active ingredient of the specific vaccine or other information that might enable consumers to identify the particular vaccine or the manufacturer of the vaccine
- statements or the implication that harmful effects will result from not receiving the vaccine
- statements or the implication that the vaccine offered is superior to other vaccines (eg a statement about the efficacy against a particular strain)
- incentives to encourage a consumer to obtain the service or vaccine
- any comparisons between vaccines (even if supported by evidence).
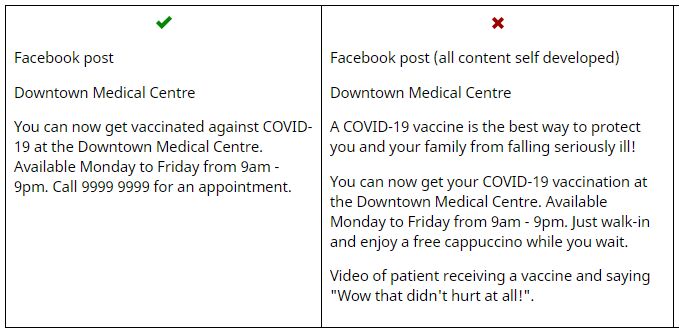 Examples of appropriate (L) and inappropriate (R) COVID vaccine advertising messages. (Image: TGA)
Examples of appropriate (L) and inappropriate (R) COVID vaccine advertising messages. (Image: TGA)
Meanwhile, the TGA has warned against using advertising materials in a way that may alter the take-home message for patients, through either ‘placement of the materials or proximity to other promotional materials’.
‘The purpose of these guidelines is provide additional clarity to healthcare professionals on the existing protocols for proactive communications and advertising for prescription medicines and vaccines,’ a TGA spokesperson told
The Canberra Times.
‘Vaccines are prescription medicines and therefore ordinarily cannot be advertised to consumers.’
When it comes to conversations with patients and use of social media, Dr Price says the key requirement is that it is based on science.
‘My understanding is that you are able share accurate scientific information on the vaccines via social media,’ she said.
‘[However], GPs will not be able to add any further commentary in their post that advertises for people to come to their practice to receive the jab. But they can talk about scientific material.
‘The TGA is recommending that practices specifically use materials developed by the government, such as posters, that do not contain information about the brand of vaccine.’
The move follows months of public debate, comparing the Oxford University/AstraZeneca and Pfizer/BioNTech vaccines.
‘There have been a lot of comments circulating about “superior vaccines”, and so the TGA is taking precautions to ensure there is no misunderstanding around efficacy and safety that could impact uptake or risk breaching the ban on advertising prescription medications,’ Dr Price said.
However, Dr Price added that GPs will need further guidance than is currently available around the nuances of the restrictions.
‘I was assured that these important clarifications will be forthcoming from the TGA,’ she said.
‘[I] welcome the opportunity to provide feedback and work together on this important vaccine rollout.’
Log in below to join the conversation.
coronavirus COVID-19 TGA Therapeutic Goods Administration vaccine advertising vaccines