News
Australia has secured a COVID vaccine candidate. What happens next?
With around 24 million people requiring a dose, questions remain over when and how it will be delivered, who will get first access, and if it will even work at all.
 AZD1222 is undergoing phase 3 testing in various countries and could be ready by the end of the year. (Image: AAP)
AZD1222 is undergoing phase 3 testing in various countries and could be ready by the end of the year. (Image: AAP)
The vaccine candidate, Oxford University and AstraZeneca’s AZD1222 (previously known as ChAdOx1 nCoV-19), is currently undergoing phase 3 testing in various countries and is widely expected to be one of the first vaccines available for use against COVID-19.
Should clinical trials prove successful, there is a chance the vaccine could be available in a matter of months, but the cost and where it will be manufactured in Australia is not yet clear.
These details aside, Australian Prime Minister Scott Morrison has indicated the Federal Government will target a 95% vaccination rate if and when a candidate becomes available.
According to Associate Professor Mark Morgan, Chair of the RACGP Expert Committee – Quality Care (REC–QC), such an undertaking will require massive amounts of preparation to avoid issues witnessed in other mass-vaccination programs, such as seasonal influenza shots.
‘Each year the seasonal influenza vaccine is announced with a media fanfare – we drive up expectations and then disappoint people by delays in distribution,’ he told newsGP.
‘The demand for a COVID vaccination will be hugely greater than seasonal influenza. There will need to be a lot of planning for the distribution, storage, delivery and post-vaccination safety monitoring.’
The CSIRO’s Australian Centre for Disease Preparedness (ACDP), which took part in a pre-clinical study of AZD1222, is currently evaluating different administration methods of the vaccine to determine whether an injection or nasal spray provides better protection.
But whether it is delivered via nasal spray or injection, Associate Professor Morgan said GPs are ‘very likely’ to be heavily involved in the vaccine’s distribution.
‘GPs will have central roles in answering questions about COVID vaccination when, and if, a successful vaccine becomes available,’ he said.
‘Planning needs to be in place to fund this activity because Medicare currently excludes fee-for-service rebates for mass immunisation campaigns.’
The Federal Government’s COVID-19 vaccine and treatment strategy indicates that the Australian Technical Advisory Group on Immunisation (ATAGI) is preparing advice to support planning for the allocation and use of any coronavirus vaccine, with the topic on the agenda for its next meeting, due to take place this week.
The Government’s strategy also involves the development of a monitoring and surveillance plan designed to ensure ongoing safety, while Commonwealth agencies are working with states and territories on transportation, storage and distribution plans.
Associate Professor Morgan said if vaccination becomes available at multiple sites, such as community pharmacies, government vaccination centres, hospitals, or workplaces, a robust system for recording who has and who has not been vaccinated will be crucial.
‘We know this is patchy for seasonal influenza immunisation,’ he said.
‘There will [also] need to be clear information available about how long it takes to become immune after the vaccination and how this changes the need for avoiding exposure to COVID-19.
‘Some of the public health messaging has been very black and white despite major scientific uncertainties – just think of how the messaging about mask use or school spread has changed over time.
‘It is going to be very important for public confidence to be clear, from the start, about the level of certainty and uncertainty surrounding any new vaccine.’
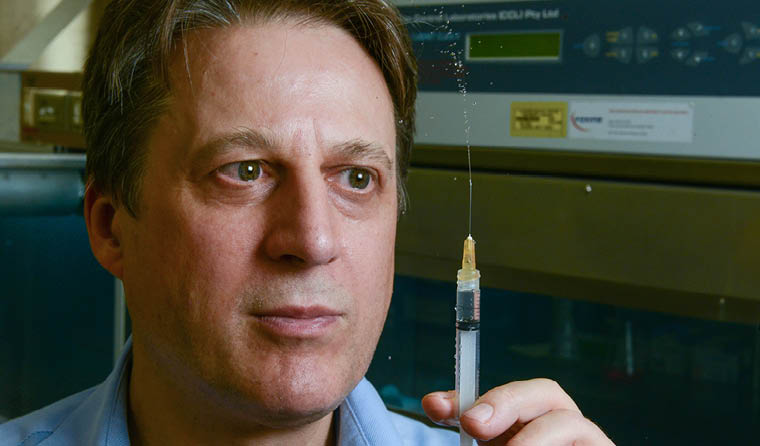 Flinders University Professor Nikolai Petrovsky is leading clinical trials for COVAX-19, another promising coronavirus vaccine candidate.
Flinders University Professor Nikolai Petrovsky is leading clinical trials for COVAX-19, another promising coronavirus vaccine candidate.
While the overall cost of the vaccinating 95% of the population has not been quantified, $24.7 million has been spent securing 100 million needles and syringes from the US and chief political correspondent with The Conversation, Michelle Grattan, estimates it will eventually surpass ‘billions of dollars’.
By way of comparison, AstraZeneca has already agreed to supply the US with 300 million doses of its vaccine for $1.66 billion, or around $5.50 per dose.
Such a price would appear to be on the cheaper end of the vaccine scale, with US pharmaceutical companies such as Moderna and Pfizer hoping to generate profit with their candidates, resulting in price ranges from $54–83 per dose.
However, the Australian Government’s COVID strategy is not restricted to one vaccine, and $256 million has been invested in the development of candidates around the world.
Yet while some of this funding has gone towards promising local candidates, such as the University of Queensland’s ‘molecular clamp technology’ vaccine, others, like Flinders University’s COVAX-19, are yet to receive any federal support.
Regardless of the successful candidate, Associate Professor Morgan said it is vital that clinical testing occurs across the spectrum of people who are likely to receive the vaccine, as well as those who may already have been exposed to the virus.
‘This includes people who are often excluded from early trials – the young, the old, pregnant, breast feeding and people with multimorbidity or impaired immunity,’ he said.
‘The participants in the vaccine safety trials [for ADZ1222] had no history of COVID-19 infection. It will be important to test the vaccine in those who have recovered or have serological evidence of COVID-19 in case there are increased side effects in these people who are already sensitised to the virus spike protein.’
Log in below to join the conversation.
coronavirus COVID-19 general practice immunisation vaccine
newsGP weekly poll
Health practitioners found guilty of sexual misconduct will soon have the finding permanently recorded on their public register record. Do you support this change?