News
Vaccine search: Australian study moves to phase 2 trials
As COVID-19 claims more Australian lives and authorities consider longer and harsher lockdown restrictions, newsGP speaks with a leading researcher about the prospect of a vaccine.
 COVAX-19 is made from a synthetic protein using a plant sugar, and is based on an earlier SARS-1 coronavirus vaccine that proved effective in animal models.
COVAX-19 is made from a synthetic protein using a plant sugar, and is based on an earlier SARS-1 coronavirus vaccine that proved effective in animal models.
The Central Adelaide Local Health Network Human Research Ethics Committee has given the green light for the vaccine, dubbed COVAX-19, to begin phase 2 trials after preliminary safety data from phase 1 found it to be safe and successfully generate an immune response.
Led by Flinders University Professor Nikolai Petrovsky, founder of biotechnology firm Vaxine, the randomised trial is being conducted at the Royal Adelaide Hospital and involves 40 healthy volunteers aged 18–65.
Thirty participants received two doses of the vaccine three weeks apart, and 10 were given a placebo.
‘The subjects have had the vaccine, they’ve had no problems at all, and so that now allows us to move forward into much bigger studies to confirm the effectiveness of the vaccine,’ Professor Petrovsky told newsGP.
‘There’s a whole lot of different groups in the community that behave differently, there’s the elderly, there’s young children, there’s people with chronic disease and cancer.
‘So basically we have to progressively do studies in all of those groups.’
The news comes as Victoria recorded its second-highest day of coronavirus cases on Friday, with 627 new cases. Eight more people in the state have died, including two men aged in their 50s, taking the national total to 197.
After undertaking door-to-door contact tracing of 500 people, Australian Defence Force and health authorities discovered on Thursday that around one in four people with the virus in Victoria are not staying home. More than 100 cases have been referred to police.
Victorian Premier Daniel Andrews said Friday’s case numbers were from tests collected on Wednesday, marking the half-way point of the six-week lockdown in Metropolian Melbourne and the Mitchell Shire.
Following crisis talks with Prime Minister Scott Morrison on Thursday night, Premier Andrews said experts will spend coming days analysing infection data to assess whether further steps – including longer and/or harsher lockdown restrictions – are needed to help flatten what has been a stubborn virus curve.
‘We cannot open up with these numbers, we cannot open up with significantly less than these numbers,’ Premier Andrews said.
‘The best advice may well come to me that we need to take further steps, that the steps we’ve taken are not enough to pull this up.’
The state’s Chief Health Officer Brett Sutton said stage three restrictions are having an effect, but confirmed a New Zealand-style lockdown, which saw all businesses except for essential services close, is being explored.
‘There are really significant consequences in what you do in terms of increased restrictions. It has to be focused on the data,’ he said.
To help keep numbers down, people of the Muslim faith who are observing Eid al-Adha have been urged not to gather in large groups this weekend.
NSW, however, has granted an exemption for 400 people to gather at a mosque in Western Sydney to celebrate the Islamic holiday.
NSW recorded 21 new cases on Friday, while Queensland recorded one.
As Victoria continues to record deaths among aged-care residents, Professor Petrovsky says he has offered to include those at risk of contracting COVID-19 into the next phase of his clinical trial.
‘We’ve made the offer to Victoria,’ he told The Australian.
‘Obviously our vaccine is still under testing, it would have to be done within a clinical trial but there’s no reason you couldn’t enrol people in Victorian nursing homes into the trial and give them the vaccine which would hopefully then protect them.
‘We know it’s not going to hurt because we now know that the vaccine is completely safe.’
COVAX-19 is made from a synthetic protein using a plant sugar, and is based on an earlier SARS-1 coronavirus vaccine that proved effective in animal models.
‘So it doesn’t involve any viruses,’ Professor Petrovsky said.
‘The vaccine is just a protein so it can’t hurt you, and that’s why it’s so safe.
‘We insert the gene for the spike protein from COVID-19 into insect cells that are grown in culture and secrete the synthetic protein into the broth in which the cells live. We then purify the protein from the broth to make it extremely pure and then mix this with some plant-based and synthetic sugars to make the vaccine.’
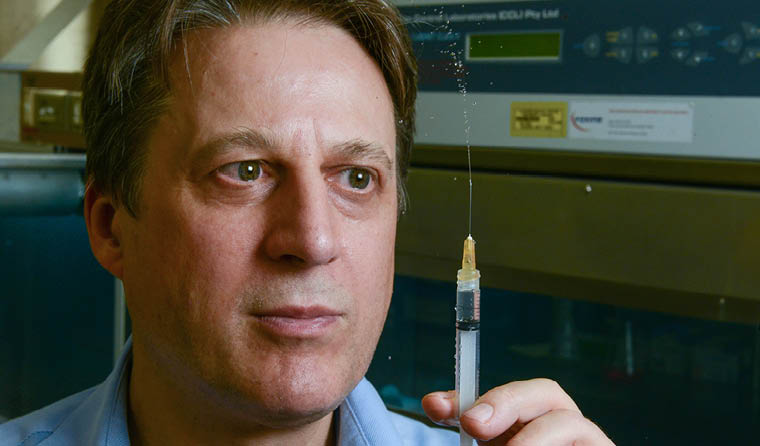
Flinders University Professor Nikolai Petrovsky is leading the randomised trial, which is being conducted at the Royal Adelaide Hospital and involves 40 volunteers aged 18–65.
Studies have emerged showing that antibodies from COVID-19 have a short life span, raising questions over long-term immunity.
Professor Petrovsky says unlike the virus itself, the vaccine is designed to induce antibodies that ‘stay around for a long time’.
‘We’ve shown that with SARS, in monkeys and in other models,’ he said.
‘So unlike natural infection, our antibodies do stick around. But, as well as that, we induce very strong T cell responses, and T cell responses generally last for the life of the individual.
‘So that provides us with a lot of confidence that the vaccine will actually protect you for a lot longer than even having the infection itself. Obviously, we still have to confirm that, but all our evidence supports that.’
Associate Professor Paul Griffin, Director of Infectious Diseases at Mater Health Services, welcomed the news, but issued a word of caution given the data has yet to be published.
‘It was … a rather small phase 1 study and when talking about immune responses only an antibody response has thus far been mentioned and only systemic side effects have been mentioned as not being present,’ he said. ‘So I would also eagerly await information relating to other immunological studies, as well as local side effect rates.
‘Overall, however, it is great to hear that the small phase 1 trial of this vaccine has generated sufficient data to approve it being utilised in further studies.’
There are currently more than 150 vaccines for COVID-19 in development globally, and COVAX-19 is one of 21 undergoing human trials.
Phase 2 trials are slated to commence in September, and will involve 400–500 volunteers.
Unlike the flu vaccine, Professor Petrovsky said, based on his experience with SARS, that he anticipates the vaccine for COVID-19 would be more like that for hepatitis B, where the individual receives an initial course, followed by a booster ‘every five to 10 years’.
If all goes well, the vaccine could be widely available by the end of this year, or early 2021, Professor Petrovsky said.
‘We’ve been doing pandemic vaccine development for 20 years now, so this is not new to us,’ he said.
‘We had the first swine flu vaccine in the world, in fact, in humans in 2009 in under three months, which was a world record at the time. We developed the SARS vaccine many years ago that was shown to be very effective in animal models.
‘And so we were able to use all that approach to make a COVID vaccine, and that’s why we’ve been able to be so fast.’
Log in below to join the conversation.
coronavirus COVID-19 lockdown vaccine
newsGP weekly poll
Which of the following areas are you more likely to discuss during a routine consultation?