News
COVID-19 vaccines and children: What GPs need to know
The TGA has paved the way for COVID-19 vaccination in children aged 5–11 from early next year. What part will general practice play?
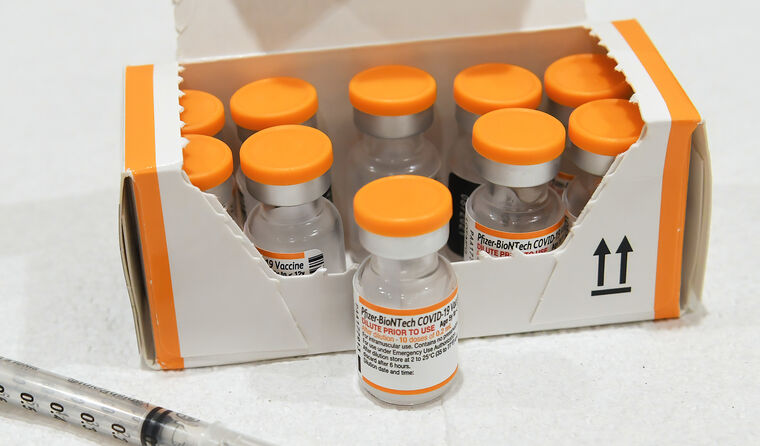 The children’s doses will be dispensed from orange-capped vials to differentiate them from adult doses. (Image: AAP)
The children’s doses will be dispensed from orange-capped vials to differentiate them from adult doses. (Image: AAP)
General practice is expected to play a key role in vaccinating young children after the Therapeutic Goods Administration (TGA) confirmed its provisional approval for Pfizer among 5–11-year-olds.
The Department of Health (DoH) said that GPs, along with Aboriginal Health Services, community pharmacies and state and territory clinics, are expected to be integral to the rollout, with full details to be confirmed following a recommendation from the Australian Technical Advisory Group on Immunisation (ATAGI).
How did the TGA reach its decision?
The TGA said the decision took into account data from clinical trials in the United States, Finland, Poland and Spain with participants in the relevant age group.
‘The study demonstrated effectiveness by showing that the immune response to the vaccine in children was similar to that seen in older age groups,’ the TGA statement reads.
Clinical trials also showed that the safety profile in children is similar to adults, with mostly mild side effects.
Professor Robert Booy, an infectious diseases paediatrician and vaccine expert at the University of Sydney, said the approval would have been informed by data emerging from places such as the United States where vaccination has already opened to younger children.
He said the data would likely have reassured health authorities about previous concerns about safety – in particular regarding myocarditis.
‘The concern we had about a signal of myocarditis in teenagers, and potentially being worse in children has not seemingly eventuated,’ Professor Booy told newsGP.
‘For the FDA [Food and Drug Administration in the US], and the TGA to be recommending a vaccine, there must be data from the real world [of the] safety in millions of children aged 5–11, indicating that myocarditis is not a serious concern.’
There have been around five million doses delivered to children aged 5–11 across the United States since Pfizer gained approval there last month.
The reasons for vaccination
Professor John Skerritt, the TGA’s national manager, said there are a number of reasons for opening up the vaccination program for the youngest age group yet. While children are broadly viewed as largely unaffected by COVID-19, Professor Skerritt highlighted what he called ‘sobering statistics’ for the cohort.
‘A bit over a fifth of all cases of COVID are actually in the under-12s,’ Professor Skerritt told reporters on Sunday.
‘And, indeed, some of the earlier data with Omicron suggests that that may actually be higher for the Omicron variant.
‘So our under-12s, who are currently unvaccinated, do catch COVID. Now, while most kids to get a fairly mild infection, and only a limited number end up in ICU … there are bigger impacts.
‘Unfortunately, about one in 3000 of the kids who get COVID actually end up with this immunological condition called multi-system inflammatory condition.
‘And those kids can end up being very sick for months. It’s not the same as long COVID, but it has some things in common. And it has a whole range of symptoms, where the kid is just not well, and that’s one of the things we’re protecting against by vaccinating children.’
Dr Ketaki Sharma is a general paediatrician and staff specialist at the National Centre for Immunisation Research and Surveillance (NCIRS). She believes the rare, but potentially serious condition of Multi-System Inflammatory Condition (or PIMS-TS; paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2) has been under-reported.
‘There are clear direct benefits to the child in preventing [infection], but I think a lot of those issues haven’t necessarily been publicised as much,’ Dr Sharma told newsGP.
‘The general media message may have been that “COVID is mild among children”, but they can get severe illness and they can get this complication.’
Professor Booy also agrees COVID-19 could pose a risk for healthy children.
‘They are at much lower risk of serious disease,’ he said. ‘But about half of [paediatric COVID-19] deaths in the UK are in previously well children, so although there’s a small risk, it’s measurable.’
Professor Skerritt said trying to ensure as normal a life as possible for children – socially, at school, and for sporting activities – in the face of a pandemic is another part of the equation.
‘The ability to vaccinate those kids so that they can return to those activities [and] their parents can be confident they can return to those activities, is a real step ahead,’ he said.
He also believes cutting the onward transmission rate is another consideration, saying that an estimated two-thirds of children transmit the virus back to their families.
How will the rollout for this age cohort be different?
The vaccine, which will not be mandatory, will be supplied through paediatric doses of 10 micrograms, a third of the dose issued to older age categories.
It will also be packaged differently to the current vaccine approved for people 12 and over. According to the TGA, the children’s doses will be dispensed from orange-capped vials instead of grey or purple capped vials.
Dr Sharma, meanwhile, says GPs are likely to face more questions on the risks and benefits of vaccination in this cohort.
‘Particularly given the age group, the fact that parents will be making the decision, they’re likely to want to have a conversation about vaccination before going ahead,’ Dr Sharma said.
‘Having that relaxed setting, [with the GP] potentially knowing the child, is likely to be much more conducive to successfully vaccinating the child.
‘That’ll be a key part of the rollout.’
Another potential challenge is a greater possible prevalence of needle phobia compared to the population as a whole, an issue raised last month by Associate Professor Margie Danchin of the Murdoch Children’s Research Institute.
‘We know among the adolescent group that needle phobia is huge … and I suspect that will become a big issue in primary school children as well,’ she previously told newsGP.
Dosage interval
Regulatory approval has been given for a three-week interval between doses, although Professor Skerritt has said that ATAGI will consider data coming in from other countries and assess whether a longer gap can produce a stronger immune response.
What is the latest with the 12–15 year-old vaccinations?
In September, the vaccination rollout was opened to 12–15-year-olds, with more than three in every four of that age group now having received at least one dose of their primary vaccination course. Federal Health Minister Greg Hunt said on Sunday (5 December) that 76.7% of the cohort had received a first dose while 67.8% had received two vaccine doses.
When will bookings open for 5–11 year-olds and how many are eligible?
There are an estimated 2.3 million children in the 5–11 age bracket, the DoH has said. The first shipment of the Pfizer paediatric doses is due to arrive in Australia in early 2022, and bookings are expected to open after ATAGI issues its recommendation.
The provisional start date has been set for 10 January.
Will other vaccines be available for younger children?
Pfizer is the only COVID-19 vaccine to have gained provisional regulatory approval so far. However, the TGA also confirmed an application from Moderna for approval for its COVID-19 vaccine for children aged 6–11 is currently being assessed. Note that the minimum age in the Moderna application is a year older than for the Pfizer vaccine.
The NCIRS is holding a webinar from 12pm – 1.30 pm (AEDT) on Wednesday 8 December covering vaccines and COVID-19 among children and adolescents, including the latest data on transmissions. See the NCIRS website for more information.
Log in below to join the conversation.
children COVID-19 paediatric vaccination Pfizer vaccine rollout
newsGP weekly poll
Which of the following areas are you more likely to discuss during a routine consultation?