News
Important psoriatic arthritis drug listed on the PBS
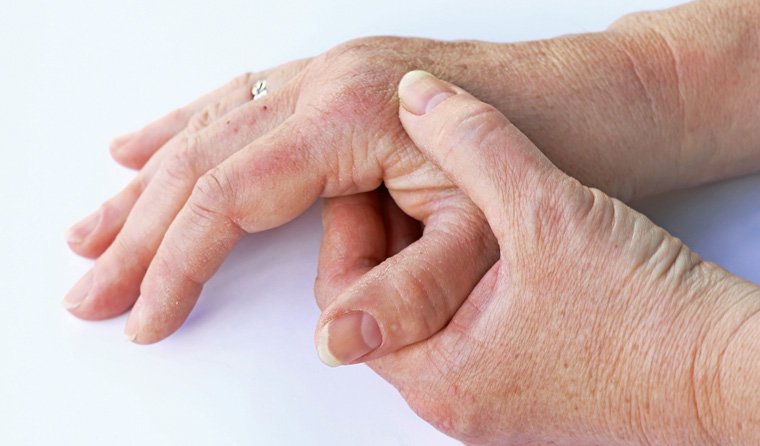
Ixekizumab is available for patients with the life-long condition, which causes joints to become stiff, painful and often swollen.
 The drug’s listing reduces the cost of a standard script to $40.30, or $6.50 for holders of a concession card.
The drug’s listing reduces the cost of a standard script to $40.30, or $6.50 for holders of a concession card.
Prior to the drug’s listing on the Pharmaceutical Benefits Scheme (PBS), patients had been expected to pay approximately $3400 per script or $19,600 per course of treatment.
However, its inclusion in the scheme reduces the cost of a standard script to $40.30, or $6.50 for holders of a concession card.
The interleukin-17A (IL-17A) inhibitor, sold as Taltz, can be used on its own or in combination with conventional antirheumatic drugs in adult patients who have responded inadequately or who are intolerant to one or more therapies.
Psoriatic arthritis most commonly co-exists with psoriasis and it is estimated that around one in eight people with the skin disease will also develop the often-painful inflammation.
Associate Professor Peter Nash, from the University of Queensland’s Department of Medicine, described the listing as welcome news for patients with the debilitating condition.
‘Joint damage can be progressive in psoriatic arthritis, with increased disability and reduced quality of life. It is important that patients have access to alternate therapies to help improve disease control,’ he said.
‘We know that half of patients with active psoriatic arthritis, including those with mild psoriasis, actually consider their psoriatic arthritis to be severe, highlighting the negative impact of both rash and arthritis.
‘It is important to consider therapy for both joint and skin symptoms in relevant patients, to help improve symptom control and quality of life.’
Ixekizumab, which is administered by subcutaneous injection via an auto-injector, has been available on the PBS to patients with severe plaque psoriasis since February 2017.
As such, it provides an alternate treatment option for patients with active psoriatic arthritis and co-existent psoriasis, to treat both joint and skin symptoms.
The PBS listing as a treatment for patients with active psoriatic arthritis was based on the results of two phase III, multicentre, randomised, double-blind, placebo-controlled that involved more than 745 patients.
As ixekizumab is a medicine that affects the immune system, it may lower patients’ ability to fight infections and increase the risk of infections, which can sometimes become serious.
Side effects potentially include severe allergic reactions and Crohn’s disease or ulcerative colitis, while common adverse events include injection site reactions, upper respiratory infections, nausea and fungal infections.
PBS Pharmaceutical Benefits Scheme psoriasis psoriatic arthritis
newsGP weekly poll
Would it affect your prescribing if proven obesity management medications were added to the PBS?