News
Remdesivir under review by national COVID-19 taskforce
newsGP speaks to taskforce Chair Associate Professor Julian Elliott about the latest guidance for the care of coronavirus patients.
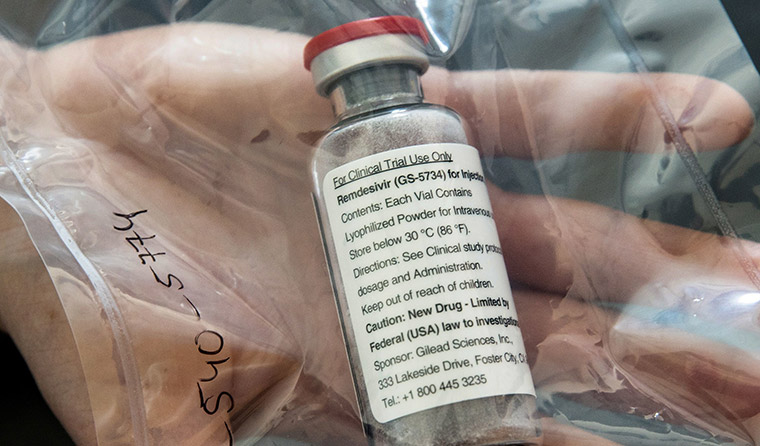 Currently, remdesivir souhld only be used in randomised clinical trials with appropriate ethical approval. (Image: AAP)
Currently, remdesivir souhld only be used in randomised clinical trials with appropriate ethical approval. (Image: AAP)
Remdesivir is among the drugs being touted as a possible treatment in the fight against COVID-19.
But in its latest update, the National COVID-19 Clinical Evidence Taskforce (the taskforce) remains cautious and preliminary studies into the drug’s effectiveness remain mixed.
One study, a small Chinese clinical trial published in The Lancet, found the drug was not associated with statistically significant clinical benefits.
However, a more recent preliminary report from the Adaptive COVID-19 Treatment (ACTT) trial shows it may have some promise. The ACTT’s interim results from the double-blind, randomised, placebo-controlled trial involving 1063 patients indicated some improvement in the time to recovery with no definite effect on mortality.
But despite the findings, Associate Professor Julian Elliott told newsGP clinicians don’t have the go-ahead just yet, with the taskforce still recommending that remdesivir only be administered in the context of randomised trials with appropriate ethical approval.
‘What we’ve been doing is pulling those files and that evidence has been presented to our panels and is currently going through the process,’ he said.
‘It’s now going to the Guideline Leadership Group tonight and then we’d expect some revisions to the recommendation to be released later this week.’
For adults with severe to critical COVID-19, the consensus recommendation is to consider increased-dose venous thromboembolism (VTE) prophylaxis dosing of anticoagulants, such as low molecular weight heparin (LMWH), unless there is a contraindication.
This is to help prevent blood clot-related events such as stroke, pulmonary embolism and deep vein thrombosis.
The recommendation follows the emergence of case series and cohort studies around the world that reported much higher rates of life-threatening thrombosis in people with COVID-19.
‘Standard use of anticoagulants does not appear to be sufficient to prevent VTE in these patients,’ Associate Professor Elliott said.
‘While we don’t yet know what the optimal dosing regime is, because there are no trials published to date that have looked at this specifically in COVID-19, experts within the taskforce agreed that the potential benefits outweigh the known harms of higher-dose anticoagulants, such as increased risk of bleeding.’
For adults with COVID-19 requiring mechanical ventilation, the taskforce has made a conditional recommendation against the use of continuous infusions of neuromuscular blocking agents (NMBAs).
‘The panel is restating the fact that this shouldn’t be routine,’ Associate Professor Elliott said.
‘There was some initial interest that they might have a particular role in COVID-19. But subsequent to that, the feeling has been there’s really not data to support that.’
Other clinical questions currently under review include:
• safety of hydroxychloroquine (high priority)
• timing of mechanical ventilation
• tracheostomy
• use of corticosteroids
Recommendations specifically for children and adolescents, and pregnant and perinatal women with COVID-19 are expected over the coming weeks.
As states and territories begin to ease social distancing measures, there has been speculation over fears of a deadly second wave.
Whether that will be the case remains to be seen, but Associate Professor Elliott says both healthcare professionals and the general public can take solace in preparations that have been made. One of the pillars for the Government to be able to successfully reopen the economy is health system preparedness for any potential increase in cases, and the taskforce is part of that plan.
‘As a taskforce we’re very much in a mode of preparedness – we have been for some weeks,’ he said.
‘I don’t think there’s a sense of panic or anxiety, just a sense that there’s important work to be done to make sure that we’ve got these guidelines in place for all questions that clinicians have.’
To date, more than 5000 articles have been published on COVID-19, with more than 1100 clinical trials underway.
‘Australia’s got a very robust process for reviewing that enormous increase in COVID-19 research,’ Associate Professor Elliott said.
‘That mechanism is not only of high quality but can move quickly so that we know we can have the best possible guidelines so clinicians can feel supported and can provide the best possible care.
‘No other country in the world has the capacity to do this as rapidly as we can here in Australia.’
However, despite these efforts, Australia has not been immune to a rise in conspiracy theories.
In a pandemic environment, Associate Professor Elliott says it highlights more than ever the importance of GPs and other health professionals engaging in dialogue with their patients both one-on-one, and on a larger scale, to help ease any anxieties and emphasise the value of an evidence-based approach.
‘It’s important that the way we communicate about treatments to the public continues to evolve,’ he said.
‘The whole way that information is produced and consumed obviously has changed. So we do need to be out in public spaces and on social media and in the media generally so that people understand just really clearly what we know and what we don’t know.
‘We’re always going to have conspiracy theories and people feeding into that. But there’s a lot we can do to counteract those messages.’
GPs are encouraged to submit their clinical questions and provide feedback on the taskforce website.
The RACGP has more information on coronavirus available on its website.
Log in below to join the conversation.
clinical guidelines coronavirus COVID-19 remdesivir
newsGP weekly poll
Health practitioners found guilty of sexual misconduct will soon have the finding permanently recorded on their public register record. Do you support this change?