News
TGA approves vaccine booster for primary school age children
Pfizer’s COVID-19 vaccine has been given the green light as a booster for children aged 5–11, with ATAGI recommendations to follow.
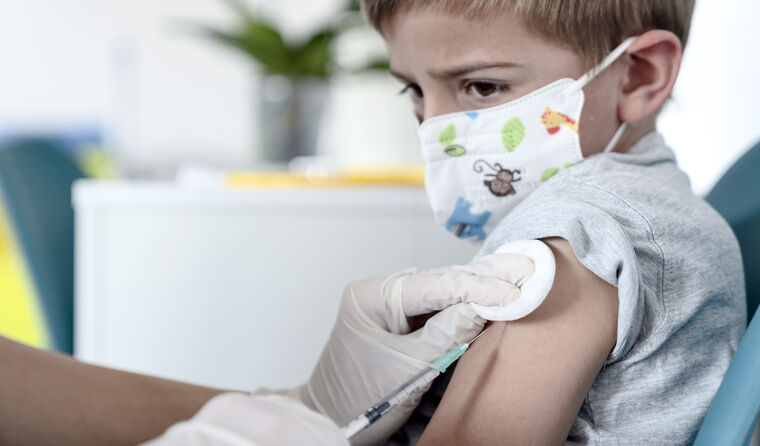 ATAGI is expected to provide specific advice for boosters in children shortly.
ATAGI is expected to provide specific advice for boosters in children shortly.
The Therapeutic Goods Administration (TGA) has provisionally approved the use of the Pfizer COVID-19 vaccine for use as a booster in children aged 5–11.
It is the first COVID-19 vaccine to be approved by the TGA for use as a booster in that age group, with the Australian Technical Advisory Group on Immunisation (ATAGI) expected to outline its specific recommendations in a few weeks.
Currently only those categorised as severely immunocompromised are eligible for a third dose, which is classified as part of the primary course.
According to the latest statistics, just over 51% of children in that age group have received a first dose, with 40% having received a second dose.
The vaccine rollout was opened to primary age school children in January this year. Pfizer was the first vaccine to be approved for the cohort with two 10 microgram doses recommended for the primary vaccination course, while the Moderna vaccine received similar backing shortly afterwards.
However, Moderna is not approved for use as a booster in the age group and there is currently no outstanding application for its approval, according to the TGA website.
Pfizer’s COVID-19 vaccine has also been approved by the TGA for use as a booster among those aged 12–15.
In that age group, ATAGI is recommending it only for those who are severely immunocompromised as well as those with a disability causing ‘significant or complex health needs’, or those with ‘severe, complex, or multiple health conditions’ that increase the risk of severe COVID-19.
Everyone over the age of 16 is recommended for a booster, with around six million people who have received their primary course now due to have a further dose.
More COVID-19 vaccine information for GPs can be found on the RACGP’s website.
Full RACGP resources for GPs and patients on COVID-19 and vaccination can be found here.
Log in below to join the conversation.
ATAGI COVID-19 Moderna paediatric doses Pfizer
newsGP weekly poll
Which of the following areas are you more likely to discuss during a routine consultation?