News
TGA provisionally approves first prevention therapy for COVID-19
The combination therapy, created by AstraZeneca, has been described as another ‘significant step forward’ in the pandemic.
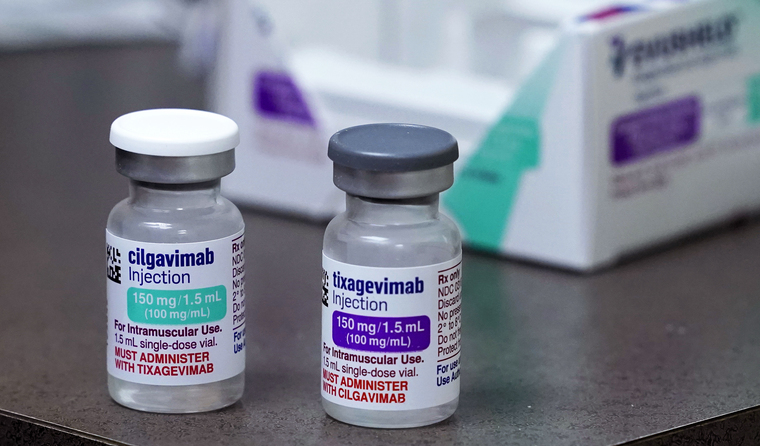 Trial data on the use of tixagevimab in combination with cilgavimab has shown an 83% reduced risk of symptomatic COVID-19. (Image: AAP)
Trial data on the use of tixagevimab in combination with cilgavimab has shown an 83% reduced risk of symptomatic COVID-19. (Image: AAP)
GPs may soon have a preventive treatment in their arsenal for COVID-19.
The Therapeutic Goods Administration (TGA) issued a statement on Friday granting provisional approval for tixagevimab and cilgavimab (sold as Evusheld), a long-acting monoclonal antibody combination therapy.
The approval is for patients who are aged 12 and older, weigh at least 40 kg and who have either:
- moderate-to-severe immune compromise due to a medical condition or receipt of immunosuppressive medications or treatments that make it likely that they will not mount an adequate immune response to COVID-19 vaccination; or
- not been recommended for vaccination due to a history of severe adverse reaction to a COVID‐19 vaccine or vaccine component.
Australia’s National COVID-19 Clinical Evidence Taskforce (the taskforce) published
clinical guidance for the use of the combination therapy on Monday.
In line with the TGA’s criteria, it does not recommend routinely using tixagevimab in combination with cilgavimab as pre-exposure prophylaxis, and instead restricts its use for ‘exceptional circumstances, in individuals who are severely immunocompromised’.
While trials have also utilised the therapy as a post-exposure prophylaxis, for people who have been exposed to COVID and are not currently positive, taskforce director Associate Professor Tari Turner told
newsGP that it is not currently recommended in Australia.
‘We’re not recommending it for use in that population – we have an “only in research” recommendation for post-exposure prophylaxis,’ she said.
‘But if a person has been exposed and has subsequently tested positive, the [therapy] can be used in that group for those patients who are at high risk of severe disease.’
The risk factors for consideration include:
- being aged 60 and over
- a higher body mass index (≥ 30 kg/m2)
- congestive heart failure
- chronic obstructive pulmonary disease (COPD)
- chronic kidney disease (eGFR < 30 mL/min)
- chronic liver disease immunocompromised state from solid organ transplant, blood or bone marrow transplant, immune deficiencies, HIV, use of corticosteroids or other immunosuppressive medicines
- intolerant of vaccine.
The taskforce does, however, note that in the
PROVENT Phase III pre-exposure prevention trial that a slightly higher proportion of individuals who received the therapy experienced a cardiovascular adverse event.
While the numbers were low, and it is unclear to what extent treatment had an impact, the taskforce does advise that ‘caution should be taken when considering tixagevimab plus cilgavimab administration in individuals with pre-existing cardiovascular conditions’.
Similar to vaccination, the therapy is administered as two separate, sequential intramuscular injections – 150 mg of tixagevimab and 150 mg of cilgavimab.
The antibodies work by binding to two different sites of the SARS-CoV-2 spike protein to block the virus from entering the body’s cells and causing infection.
Asked whether the therapy could be administered in general practice, Associate Professor Turner said that is a consideration ‘for the jurisdictions’.
As part of the approvals process, the TGA considered data from the
PROVENT Phase III pre-exposure prevention trial, which included more than 5000 participants, of whom upwards of 75% had co-morbidities reported to cause a reduced immune response to vaccination.
A six-month follow-up found those who received the therapy had an 83% reduced risk of symptomatic COVID-19, with no severe disease or deaths, compared to those in the placebo group.
The combination therapy has also been approved for use in a number of other countries, including
the US and more recently
Israel. But on Thursday (24 February), the Food and Drug Administration (FDA) revised its authorisation to
double the initial dose from 150 mg to 300 mg of both tixagevimab and cilgavimab, citing concerns the therapy may be ‘less active’ against certain Omicron subvariants.
‘Available data indicate that a higher dose of Evusheld may be more likely to prevent infection by the COVID-19 Omicron subvariants BA.1 and BA.1.1 than the originally authorised Evusheld dose,’ the FDA said in a
media release.
Asked whether this could also eventuate in Australia, due to the therapy having been trialled pre-Omicron, Associate Professor Turner said the taskforce has yet to see the data being cited by the FDA.
‘We need to wait to see that before we revise the decision, or could make a decision about whether it needed to be revised,’ she said.
Associate Professor Paul Griffin, who is the Director of Infectious Diseases at Mater Health Services and Medical Director and Principal Investigator at Q-Pharm Nucleus Network, said while Australia already has an array of safe and effective vaccines and treatments, a proportion of the population remains vulnerable who will benefit from the combination therapy.
‘In my opinion, this approval represents another significant step forward in our capability to control this virus and further limit its impact,’ he said.
Professor of Viral Immunology at Murdoch University, Cassandra Berry agrees, but highlighted that the therapy is ‘not a substitute’ for vaccination.
‘This is a form of passive immunity rather than active immunity,’ she said.
‘Pre-exposure prophylaxis with these antibodies is only effective for about six months and does not last forever. So it is a complimentary protective measure for COVID and is not a substitute for vaccination, which does induce immune memory.’
Associate Professor Turner said that it is line with the stance of the taskforce.
‘It’s great to be in a position that we now have multiple options for treatment for patients who are at higher risk of [disease] progression,’ she said.
‘But the situation still remains that vaccination is the most important form of protection for us and all of these drugs that are now being potentially recommended … they’re really only helpful treatment options for people who are unvaccinated or who are immunosuppressed or have immunocompromise.
‘So it’s really helpful for clinicians to have an expanded toolset. And each of the drugs that’s now potentially available has strengths and weaknesses and different contraindications, and so on, so having some options is useful.’
The TGA’s decision was also informed by expert advice from the Advisory Committee on Medicines, and AstraZeneca is expected to continue providing information to the regulator on longer-term efficacy and safety from ongoing clinical trials and post-market assessment.
The Australian Government has secured 36,000 treatment courses.
Log in below to join the conversation.
AstraZeneca cilgavimab COVID-19 Evusheld prophylaxis TGA tixagevimab
newsGP weekly poll
As a GP, do you use any resources or visit a healthcare professional to support your own mental health and wellbeing?