News
What is the latest on COVID-19 treatments available in Australia?
As infection rates continue to reach record highs, newsGP breaks down the latest treatments available for people who have the viral infection.
 From monoclonal antibodies to corticosteroids, there are a number of effective treatments for COVID-19 approved for use in Australia.
From monoclonal antibodies to corticosteroids, there are a number of effective treatments for COVID-19 approved for use in Australia.
It seems almost impossible, but just two years ago people around the world had never heard of SARS-CoV-2.
The novel virus has since rapidly spread across the globe, resulting in more than 290 million infections and over 5.4 million deaths.
In the meantime, however, thanks to investment from governments and philanthropists, scientists have been able to develop a considerable knowledge base around COVID-19 at great speed, and highly effective vaccines and treatments are increasingly becoming available.
As infection rates in Australia ramp up, with more than 148,000 cases confirmed in Australia over three days this week alone, newsGP provides GPs with a round-up of the country’s recommended COVID-19 treatments.
MILD-TO-MODERATE CASES
Budesonide
Budesonide is among the first-line treatments available for people with COVID-19 who develop mild-to-moderate illness.
A commonly used asthma drug, research conducted in the UK has shown that if administered early on in the infection, the corticosteroid can help prevent worsening symptoms and speed up recovery time.
Australia’s National COVID-19 Clinical Evidence Taskforce (the Taskforce) recommends inhaled budesonide be considered for use in people who contract the virus and are at risk of developing severe disease, but who do not require oxygen.
Risk factors for disease progression include people aged 50 and older with one or more of the following comorbidities:
- Diabetes (not treated with insulin)
- Heart disease and/or hypertension
- Asthma or lung disease
- Weakened immune system due to a serious illness or medication
- Mild hepatic impairment
- Stroke or other neurological problem
If deemed eligible, the treatment should be administered within 14 days of symptom onset.
Budesonide is also safe for use in pregnant or breastfeeding women, and is recommended for use in children and adolescents who do not require oxygen and who have one or more paediatric complex chronic conditions (PCCC).
These include congenital and genetic cardiovascular, gastrointestinal, malignancies, metabolic, neuromuscular, renal and respiratory conditions, as well as severe asthma or obesity.
Casirivimab plus imdevimab (ronapreve)
Casirivimab and imdevimab, also known as ronapreve, was provisionally approved for the treatment of COVID-19 by the Therapeutic Goods Administration (TGA) in October 2021.
A combined monoclonal antibody treatment,
research has shown it reduces the risk of severe infection and hospitalisation. It works to prevent the virus from infecting healthy cells by binding to distinct regions of the spike protein.
There are concerns, however, that the ronapreve may have decreased effectiveness against the Omicron variant based on in vitro data, but definitive evidence is still lacking.
At present, the Taskforce recommends the treatment be considered for use in patients who do not require oxygen and have one or more risk factors for disease progression, including:
- being aged 50 and over
- obesity (BMI ≥ 30 kg/m2)
- cardiovascular disease (including hypertension)
- chronic lung disease (including asthma)
- type 1 or 2 diabetes mellitus
- chronic kidney disease, including those that are on dialysis
- chronic liver disease
- immunocompromised (including people with rheumatoid arthritis, HIV/AIDS and systemic lupus erythematosus receiving immunosuppressive treatment).
It is recommended 1200 mg be administered within seven days of symptoms.
Ronapreve can also be considered for use in pregnant or breastfeeding women who have mild COVID-19, and in all adults hospitalised with moderate-to-critical disease who have no detectable SARS-CoV-2 antibodies.
In exceptional circumstances, the treatment can be considered for use in children and adolescents aged 12 and over who weigh at least 40 kg and who are at high risk of deterioration.
Sotrovimab
A monoclonal antibody treatment, sotrovimab was provisionally approved by the TGA in August 2021 and is
one of the leading treatments for people with mild-to-moderate COVID-19 who are at risk of developing severe disease.
In
the COMET-ICE trial, unvaccinated participants with COVID-19 who received a single one-hour intravenous infusion of 500 mg sotrovimab were found to have a 79% reduced rate of hospitalisation and death compared to those who received a placebo.
Designed to mimic the natural antibodies produced by the immune system, the monoclonal antibodies bind to the virus to stop it from entering host cells and help the body to fight already infected cells. However, questions remain regarding the treatment’s efficacy in vaccinated or immunocompromised patients.
Sotrovimab is so far among the treatments showing promise against the Omicron variant, with the Australian Government having
recently secured an additional 46,000 units, bringing the country’s total to 81,000.
The Taskforce has conditionally approved its use within five days of symptom onset for those who do not require oxygen and who have one or more of the following risk factors:
- Diabetes (requiring medication)
- Obesity (BMI ≥ 30 kg/m2)
- Chronic kidney disease (ie eGFR < 60 by MDRD)
- Congestive heart failure (NYHA class II or greater)
- Chronic obstructive pulmonary disease (history of chronic bronchitis, chronic obstructive lung disease, or emphysema with dyspnoea on physical exertion)
- Moderate-to-severe asthma (requiring an inhaled steroid to control symptoms or prescribed a course of oral steroids in the previous 12 months)
- Aged 55 and over
Clinicians can also consider sotrovimab in pregnant women who are in the second or third trimester, and in exceptional circumstances for children or adolescents aged 12 and over who weigh at least 40 kg.
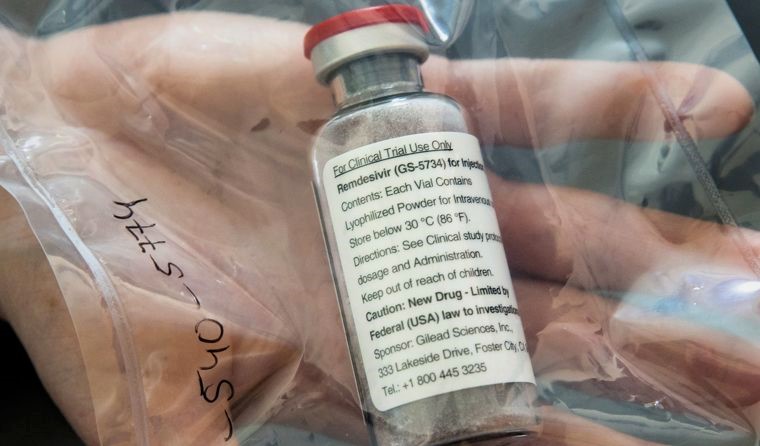 The antiviral remdesivir was the first treatment for COVID-19 to be approved for use in Australia. Image: AAP
SEVERE-TO-CRITICAL CASES
Dexamethasone
The antiviral remdesivir was the first treatment for COVID-19 to be approved for use in Australia. Image: AAP
SEVERE-TO-CRITICAL CASES
Dexamethasone
The corticosteroid was the first treatment to become available for COVID-19 that
demonstrated reduced mortality.
Specifically recommended for patients with severe-to-critical COVID-19 who are receiving oxygen, including mechanical ventilation, the Taskforce recommends 6 mg of the drug be administered intravenously or orally for up to 10 days. There are also alternative regimens for use in adults and pregnant or breastfeeding women.
The treatment can also be considered for use in children and adolescents with acute COVID-19 who are receiving oxygen, including mechanically ventilated patients.
Remdesivir
Remdesivir was
the first treatment for COVID-19 to be approved by the TGA back in July 2020, based on evidence the antiviral can reduce hospitalisation time for those experiencing severe infection.
The Taskforce recommends using remdesivir in adults with COVID-19 who require oxygen, but who do not require ventilation.
While it remains unclear whether a 5- or 10-day regimen is optimal, in Australia the criteria for access to remdesivir from the National Medical Stockpile limits the treatment course to five days.
It can also be considered for use in pregnant or breastfeeding women, but it is not recommended for children or adolescents outside of a clinical trial.
Baricitinib
Baricitinib is an anti-inflammatory drug recommended for use in adults hospitalised with COVID-19 who require supplemental oxygen.
The Taskforce recommends that 4 mg be administered orally for up to 14 days. However, for patients with an eGFR of between 30 and 60 mL/min/1.73m2, the dosage can be halved to 2 mg daily.
The treatment is currently only recommended for adults, with pregnant and breastfeeding women, and children and adolescents advised to receive the drug only as part of randomised trials.
Tocilizumab
Tocilizumab, a monoclonal antibody, is recommended for people hospitalised with COVID-19 who require supplemental oxygen, particularly where there is evidence of systemic inflammation.
Clinicians can also consider its use in pregnant or breastfeeding women, as well as children and adolescents.
The treatment is administered as a single intravenous infusion over 60 minutes, with the suggested dose dependent on the patient’s body weight. If deemed clinically appropriate, a second dose can be administered but the Taskforce recommends the decision should take into consideration its availability.
Since July 2021, there has been
a critical shortage of tocilizumab. The Taskforce recommends baricitinib be considered as an alternative, unless contraindicated.
Sarilumab
Similar to tocilizumab, sarilumab is a drug used to treat rheumatoid arthritis. It is recommended for use in patients with COVID-19 who are hospitalised and require high-flow oxygen or ventilation.
While evidence supports the idea the treatment ‘probably reduces the risk of death’, the Taskforce says uncertainty remains as to whether sarilumab affects mortality in patients who require no ventilatory support or low-flow oxygen.
Sarilumab is not recommended for use outside of randomised trials for pregnant or breastfeeding women, children and adolescents.
TREATMENTS UNDER EVALUATION
The TGA is currently evaluating the following treatments:
- Molnupiravir – an antiviral that comes in tablet form
- PF-07321332 – a treatment used in combination with ritonavir
- Tixagevimab and cilgavimab (Evusheld) – a treatment consisting of two monoclonal antibodies
The Federal Government has ordered supplies of molnupiravir and PF-07321332, which are anticipated to become available sometime this year pending TGA approval.
GPs can access a flow chart for use of disease-modifying treatments for adults with COVID-19 on the National COVID-19 Clinical Evidence Taskforce website.
Log in below to join the conversation.
baracitinib budesonide casirivimab COVID-19 dexamethasone imdevimab Omicron remdesivir sarilumab sotrovimab tocilizumab treatments
newsGP weekly poll
Are you interested in prescribing ADHD medication?