News
TGA updates post-COVID vaccine myocarditis rates
As many of Australia’s remaining vaccine-hesitant patients express concerns over rare side effects associated with mRNA vaccines, the TGA has broken down rates of myocarditis cases following Pfizer doses.
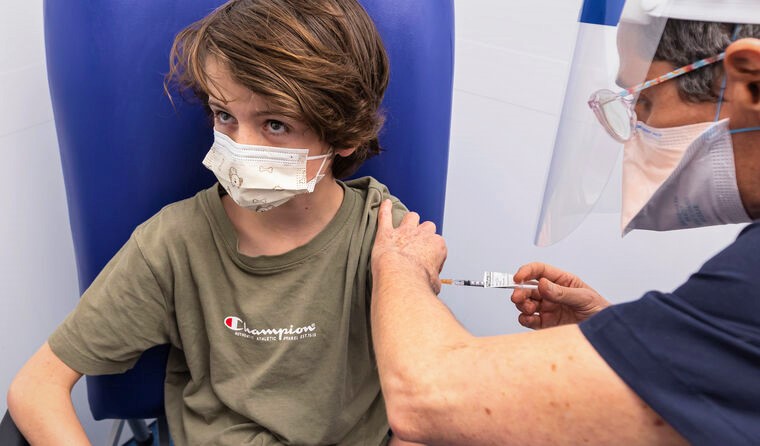 Boys aged 12–17 are more likely to develop myocarditis following their second Pfizer dose. (Image: AAP)
Boys aged 12–17 are more likely to develop myocarditis following their second Pfizer dose. (Image: AAP)
This article was updated at 5.50 pm Monday 22 November.
‘I’m worried about the side effects.’
It is a common refrain among Australians who are still reluctant to receive a COVID vaccine, whether due to the extremely rare possibility of blood clots with AstraZeneca, or myocarditis and pericarditis with Pfizer or Moderna.
As fewer doses of AstraZeneca are administered in the latter stages of Australia’s vaccine rollout, hesitant people are increasingly expressing concerns over the mRNA options.
Melbourne’s Sumit Aneja is concerned after someone he knows who had never previously had heart problems developed pericarditis following a vaccine.
‘To me COVID doesn’t present a real danger to my life because I’m a healthy individual, I take care of myself,’ he told Nine Newspapers.
‘It’s a disease, anyone can get it. But if I willingly put something in my body which can cause me harm, I’ll never be able to forgive myself.’
But according to the Therapeutic Goods Administration’s (TGA) latest safety report, the likelihood of developing myocarditis or pericarditis after an mRNA vaccine remains exceedingly rare.
Australia recorded only 28 new cases deemed ‘likely myocarditis’ following mRNA vaccination over the previous week, 10 fewer than the prior seven days, the report found.
The updated figures, current as of 14 November, mean there have been 329 reports of likely myocarditis from approximately 23.4 million doses of Pfizer and 978,000 Moderna doses.
A further 592 cases have been identified as ‘suspected myocarditis’, including 116 in children aged 12–17, while there have been 1370 cases of suspected pericarditis only.
The youngest case classified as likely myocarditis to date was 12 years old.
Being infected with COVID-19 itself is ‘associated with a substantially higher risk of myocarditis and other cardiac complications compared to the COVID vaccines’.
The relatively low number of Moderna doses administered in Australia to date means the TGA is unable to provide a reliable estimated rate of myocarditis following vaccination. However, it has provided the below table in relation Pfizer, which breaks down the rates between age groups and sexes.
Rates of myocarditis cases following Pfizer
| |
|
| Age (years) |
All doses |
Second doses |
|
| Rate per 100,000 doses |
Rate per 100,000 doses |
|
| |
Male |
Female |
Male |
Female |
|
| 12–17 |
5.6 |
1.3 |
8.5 |
2.3 |
|
| 18–29 |
3.1 |
1.2 |
3.7 |
1.5 |
|
| 30–39 |
1.4 |
0.6 |
1.5 |
0.6 |
|
| 40–49 |
0.7 |
0.8 |
1.2 |
1.1 |
|
| 50–59 |
0.4 |
0.4 |
0.1 |
0.4 |
|
| 60–69 |
0.0 |
0.4 |
0.0 |
0.0 |
|
| 70+ |
0.0 |
0.2 |
0.0 |
0.0 |
|
| All ages |
2.1 |
0.9 |
2.7 |
1.1 |
|
The table shows suspected myocarditis following a second dose of Pfizer occurs at a rate of 8.5 cases per 100,000 in boys aged 12–17, more than three times higher than the overall rate among all males of 2.7 per 100,000.
Boys aged 12–17 are also more than twice as likely to develop suspected myocarditis following a first Pfizer dose (5.6 per 100,000) than the general male population. Both of the 12–17 age group figures are an increase on the rates contained in the
most previous TGA safety report (5.2 and 7.1 cases per 100,000 first and second doses, respectively).
Importantly, the TGA states that while the rates contained in the table include cases of likely myocarditis that occurred after vaccination, they may not necessarily be vaccine-related and are still lower than myocarditis rates in people infected with COVID-19.
Additionally, the number of younger people vaccinated is still relatively low in Australia, so estimated reporting rates are based on limited data.
According to the TGA, of the cases classified as likely myocarditis, most patients experienced symptoms within three days of vaccination. Around half were admitted to hospital, including 11 who were treated in intensive care.
Most patients treated in hospital were discharged within four days and there have been no vaccine-related fatalities for either Pfizer or Moderna in Australia.
Nine people are suspected to have died following a dose of the AstraZeneca vaccine, including eight due to thrombosis with thrombocytopaenia (TTS) and one case of immune thrombocytopaenia (ITP).
However, the number of TTS cases has reduced substantially in recent months alongside reduced utilisation of the AstraZeneca vaccine, with
the most recent deaths occurring at the end of August.
Only three new probable cases of TTS were identified in the past week, all men aged 62–78, bringing the overall number of confirmed or possible cases to 163 from 13.4 million doses.
As well as TTS and ITP, the TGA is also monitoring reports of Guillain-Barre syndrome (GBS) following vaccination with AstraZeneca.
A while clear link between the vaccine and GBS has not been established, the TGA has received 148 reports of people developing the syndrome following vaccination, at a rate of about one in every 100,000 people.
Overall, nearly 81,000 adverse events have been reported to the TGA since the beginning of the rollout at a rate of about 2.1 per 100,000. The vast majority of these have been injection-site reactions (such as a sore arm) and more general symptoms, like headache, muscle pain, fever and chills.
Around 10,000 potential claims related to adverse reactions have been registered with the Federal Government’s no-fault indemnity scheme since September.
Log in below to join the conversation.
AstraZeneca COVID-19 myocarditis pericarditis Pfizer
newsGP weekly poll
Health practitioners found guilty of sexual misconduct will soon have the finding permanently recorded on their public register record. Do you support this change?