News
Will GPs be vaccinating five-year-olds against COVID by year’s end?
Preliminary results among children under 12 show promise, but larger trials will likely be needed to confirm that the benefits outweigh the risks.
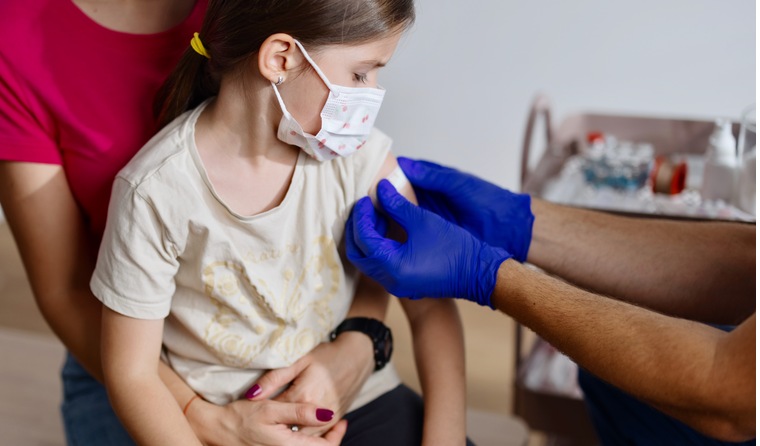 If Australia moves ahead on vaccinating 5–11-year-olds, paediatric infectious disease experts anticipate it likely will not be until next year.
If Australia moves ahead on vaccinating 5–11-year-olds, paediatric infectious disease experts anticipate it likely will not be until next year.
There could soon be a COVID-19 vaccine for children aged 5–11.
Preliminary results from Pfizer’s phase 2/3 trials were released this week and reportedly show the mRNA vaccine is safe, well-tolerated, and produces a robust neutralising antibody response.
The 2268 participants received a two-dose regimen of 10 µg – a smaller dose than the 30 µg dose used to immunise people 12 and older – administered 21 days apart.
But despite the lower dosage, researchers found the antibody responses were comparable to those recorded in the control group, who were aged 16–25 and received 30 µg doses.
One month after the second dose, the neutralising antibody geometric mean titre (GMT) for participants aged 5–11 was 1197.6 compared to 1146.5 for the control group.
Recorded side effects were also comparable to those observed in participants aged 16–25.
As a paediatric infectious disease specialist and clinician scientist at Perth Children’s Hospital, Associate Professor Asha Bowen told newsGP she is pleased to see promising data coming through for younger children.
‘It looks like the vaccine mounts an appropriate immune response in kids that would be needed to protect them from COVID-19, so it’s good news for kids and families,’ she said.
‘It’s the first step in [moving towards] the regulatory and then the approval process that will be required to figure out how to utilise this vaccine in Australian children – and hopefully we might have some more information emerging from other countries as we’re considering and thinking about those decisions.’
Professor Robert Booy, infectious diseases paediatrician and Head of the Clinical Research team at the National Centre for Immunisation Research and Surveillance (NCIRS), agrees.
However, complete data sets to support the findings have not yet been published, and Professor Booy also pointed out that while the trial determined the vaccine’s safety profile in relation to common side effects, the relatively small number of participants indicates a need for further monitoring in a larger number of children.
‘Rare side effects cannot be found by such a study,’ he told newsGP.
‘It is really important that we evaluate the experience in the real world of using vaccines in younger children in countries like the United States, where they’re likely to approve and use the vaccine sooner than Australia.’
While the Delta variant’s emergence both globally and in Australia has resulted in increased transmission of COVID-19 among children, hospitalisation and fatal cases are rare, with none recorded in Australia to date among children under 10.
For this reason, Professor Booy says vaccinating children does not make ‘nearly as big a difference as in older adults and the elderly’. But when considering the indirect benefits of vaccination, that changes.
‘The other benefits apart from preventing serious medical illness, include getting children back to school, namely education, socialisation, exercise, and mental health,’ he said.
‘Once you factor in all those elements, you get an argument in favour of child vaccination.
‘However, it is really important not to act until we have an understanding of the experience in the real world of vaccination from other countries.’
Beyond severe disease, Associate Professor Bowen says vaccinating younger children also has the potential to protect them from developing long COVID or paediatric inflammatory multisystem syndrome temporally associated with SARS-COV-2 (PIMS-TS).
Pfizer and BioNTech have said they plan to submit their data to the US Food and Drug Administration (FDA), European Medicines Agency (EMA) and other regulators ‘with urgency’ before the start of winter in the northern hemisphere.
Federal Health Minister Greg Hunt has reportedly written to Pfizer Australia’s Managing Director Anne Harris, encouraging the pharmaceutical company to apply to the Therapeutic Goods Administration (TGA) at the same time as the FDA.
‘I am heartened by your advice that trials of the Pfizer vaccine are showing promise in children under the age of 12,’ Minister Hunt wrote.
‘I note the announcement that Pfizer International is now intending to apply for regulatory approval of Pfizer’s COVID-19 vaccine for children aged 5–12.
‘I encourage and invite Pfizer to submit a parallel application to the TGA for Australian regulatory approval at the earliest possible time.’
However, Professor Booy is adamant that Australia can rely on the benefit of time and experience from abroad.
‘And therefore be confident when we look to start [vaccinating 5–11-year-olds] that we already know that the vaccine has been safely implemented in millions of children overseas,’ he said.
In the meantime, Professor Booy said Australia could potentially look to offer Pfizer to everyone over 12, including over 60s, ‘so that there is equity of access to COVID vaccines whatever your age’.
As to whether GPs are likely to be vaccinating under-12s by the end of the year, Professor Booy anticipates it won’t be until at least 2022.
‘My view is that there are enough at-risk people who still aren’t vaccinated that we have to put maximum effort into getting at risk people vaccinated this year,’ he said.
‘And young children, who might [eventually] be vaccinated, can wait in the queue until being offered vaccines next year.’
Pfizer’s phase 2/3 trials are also testing the mRNA vaccine in children aged 2–5 and 6 months–2 years.
Results are expected later this year.
Log in below to join the conversation.
children COVID-19 Pfizer vaccines
newsGP weekly poll
Which of the following areas are you more likely to discuss during a routine consultation?