News
RACGP in talks with Federal Government over COVID vaccine rollout
President Dr Karen Price gives newsGP a detailed update on logistics and funding, assuring GPs they will be a vital part of the process.
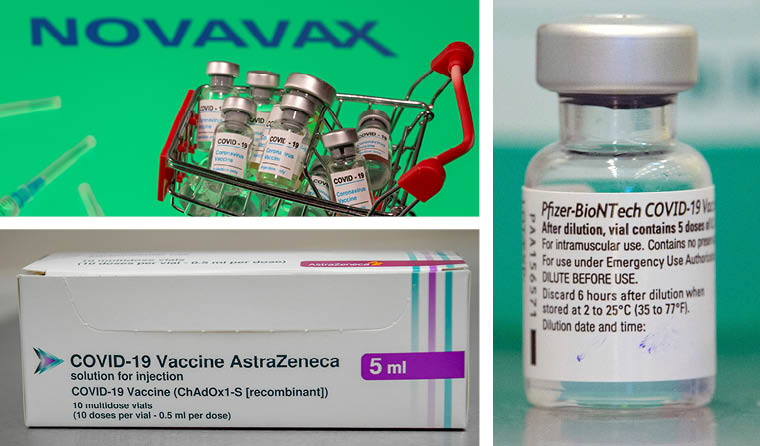 The rollout will likely commence with the Pfizer/BioNTech candidate, followed by Oxford/AstraZeneca and Novavax, pending TGA approvals.
The rollout will likely commence with the Pfizer/BioNTech candidate, followed by Oxford/AstraZeneca and Novavax, pending TGA approvals.
RACGP President Dr Karen Price has confirmed that Australia’s COVID-19 vaccination program is ‘full steam ahead’ and on track to be delivered slightly ahead of schedule in early March – and GPs are set to have a leading role.
‘I’m really pleased to see there’s so much cooperation from federal through to state, through to public health, and bureaucrats’ goodwill towards general practice,’ she told newsGP on Wednesday, following talks with the Department of Health (DoH).
‘They realise what a massive undertaking this is for our country.
‘It won’t be just GPs, but certainly – particularly initially – GPs are involved and they’ve been up front and centre.’
Pending approval by the Therapeutic Goods Administration (TGA), Federal Health Minister Greg Hunt has confirmed the rollout will commence with the Pfizer/BioNTech candidate at the beginning of March, followed by the Oxford University/AstraZeneca vaccine a few weeks later.
Dr Price said the Australian Technical Advisory Group on Immunisation (ATAGI) is currently working in close consultation with states and territories to assess the best sites for distribution, with initial locations likely to be larger GP-led respiratory clinics, general practices, and Aboriginal Community Controlled Health Organisations (ACCHOs).
‘Initially there’ll be a very small number of locations because the amounts of vaccine that are coming initially are limited,’ she said.
‘But they’re going to ramp up really quickly through March.’
The process of selection, the RACGP President confirmed, is expected to be done through expressions of interest conducted at a state and territory level, similar to that already initiated by Queensland Health.
‘Expressions of interest [will be] requested from GP clinics. Now, obviously, that will suit some clinics more than others,’ Dr Price said.
‘A really key point is that they don’t want to mix the cohort of patients who are seeking COVID testing and seeking symptom clarification with those who are coming in for vaccination.
‘So there’s going to be some logistics there and they’re still discussing it.’
When it comes to storage, most general practices currently appear to be best suited for the Oxford University/AstraZeneca and Novavax vaccine candidates because they can be stored under standard refrigerated temperatures of 2–8°C. But Dr Price said the DoH has not ruled out GPs also administering the Pfizer/BioNTech mRNA vaccine, which is stored at –70°C.
‘There was an indication that for administration, particularly the ones which need –70°C, the Commonwealth will be supporting the infrastructure and additional refrigeration that might be required – particularly for regional and rural,’ she said.
‘That was the indication that I received from today’s meeting. But, again, the details need to be worked through.’
Funding was also high on the President’s agenda during her meeting with the DoH, where she questioned whether there would be a specific item number for administration of the COVID vaccine or whether it would be block funded.
‘I emphasised that for me, as a GP, a patient coming in for a COVID vaccination would need to be triaged to a COVID-safe area, and they’ll need to be educated and consented – that takes time,’ Dr Price said.
‘Then they have to have the vaccination and wait post-vaccination for observation. So that’s going to be at least 30 minutes under my care where I’m on call for any vaccination reaction, including resuscitation from anaphylaxis.
‘This is not just a quick in and out, this is something that needs 30 minutes of general practice time.
‘[The DoH] said they’re in discussion about this, [and] have no answers yet. But there’s different types of clinics, so there’ll probably be different types of arrangements. Watch this space.’
The rollout will commence with the Pfizer/BioNTech candidate at the beginning of March.
Clinics will be required to undergo a certification process to meet minimum requirements set by the TGA to be eligible.
With limited doses available in the initial rollout, current ATAGI advice suggests priority groups are likely to be quarantine workers, workers exposed to international arrivals, frontline healthcare workers, and aged care and disability care staff. They will be closely followed by those aged 70 and older, Aboriginal and Torres Strait Islander people, those with comorbidities, and workers in critical high-transmission workplaces, Dr Price said.
With more than one vaccine candidate expected to receive approval, the Government has made it clear that each clinic will only stock one candidate and people will not have a choice as to which vaccine they receive.
‘What is going to be really important is that … there’ll be a Pfizer/BioNTech clinic, there’ll be an AstraZeneca clinic and Novavax clinics and so on,’ Dr Price said, attributing the decision to safety.
‘So it’s not like going shopping where you can choose your vaccine. It’s going to be what you can get, where you can get it.’
Talks are expected to continue on Thursday, and Dr Price says GPs can expect to have further information soon.
‘In a week’s time they’ll have more information,’ she said.
‘I think we’ll be hearing from the Commonwealth in terms of their jurisdictions, and then the state will be determining and asking for expressions of interest. That’s the way I understand it will be working.
‘The coordination between the Commonwealth and state is critical, and that’s been going well. So the college is confident in the robust processes of the Department of Health, which are underway.’
Log in below to join the conversation.
coronavirus COVID-19 vaccine rollout vaccines
newsGP weekly poll
Within general practice, do you think there are barriers to providing flu vaccinations? If so, what are they?