News
Australian coronavirus vaccine rollout to have a significant advantage
Other countries are expected to begin administering doses within weeks, which experts say should provide certainty over any lingering doubts surrounding previously unused mRNA candidates.
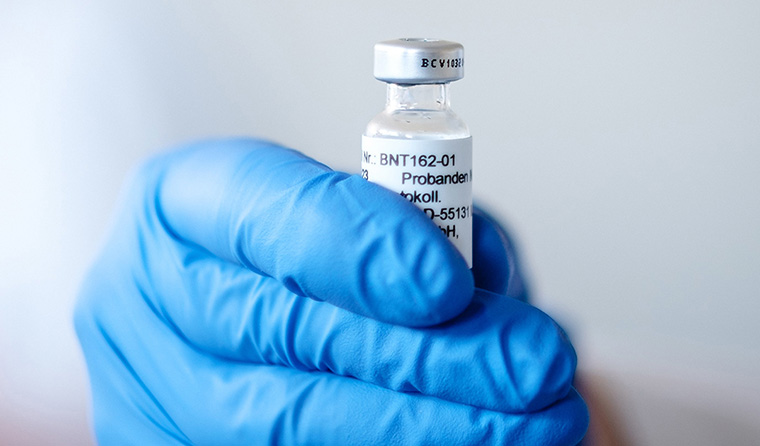 The Pfizer/BioNTech candidate represents the first time an mRNA vaccine has been licensed for routine use in humans.
The Pfizer/BioNTech candidate represents the first time an mRNA vaccine has been licensed for routine use in humans.
With the Pfizer/BioNtech vaccine candidate receiving emergency authorisation for use in the UK from next week, experts argue Australia remains in an enviable position as it waits for its own approvals.
Professor Uğur Şahin of Germany’s BioNTech recently described the vaccine candidate as potentially ‘the beginning of the end of the COVID era’. And just short of a month later, that is certainly the prevailing sentiment.
The vaccine, which has shown to be 90% effective, was granted authorisation by the UK Medicines and Healthcare products Regulatory Agency (MHRA) for emergency use on Wednesday 2 December. It is expected to be rolled out next week among specific patient cohorts, starting with frontline healthcare workers and aged care residents, followed by people aged over 80.
But the Pfizer/BioNTech vaccine also represents the first time an mRNA vaccine has been licensed for routine use in humans.
As such, while the UK’s timetable is far advanced from Australia’s own expected March 2021 rollout, University of Sydney vaccine expert Professor Robert Booy told newsGP that places Australia at a significant advantage.
‘There’s a level of uncertainty around the vaccine … and we’ll gain [clarity] from the wide implementation in the UK by way of confirming that it is effective and that it is safe,’ he said.
‘When I say “safe”, we know it’s safe already. But once it’s used in hundreds of thousands of people, we can rule out even rare side effects that occur in perhaps one in 100,000 … within weeks.’
Dr Vyom Sharma, a GP from Victoria, agrees.
Instead of relying on phase 3 trials alone, he says a real-world rollout of the vaccine will provide accurate expectations of what to expect in the next 12 months.
‘One of the questions that is not clearly answered yet is how effective the vaccines will be in preventing asymptomatic transmission of the virus – the phase 3 trials are not structured to answer this question very well,’ Dr Sharma told newsGP.
‘However, in the next few months with mass rollouts of the vaccine in nations with a high prevalence of COVID-19, we may get a clearer picture.
‘Every aspect of our COVID-19 strategy, ranging from who we vaccinate first, lifting restrictions on gatherings and workplaces, to how we can allocate resources in our healthcare systems, will be informed by these expectations.’
GPs are expected to be at the forefront of Australia’s COVID vaccine distribution and Dr Sharma said other countries’ rollouts will also be an opportunity to learn about the logistical challenges of distribution, as the Pfizer/BioNTech vaccine requires storage at -70°C.
Professor Booy anticipates there may be a need for designated immunisation ‘super clinics’, and says larger general practices may be better placed to assist with storage and distribution.
‘It’s going to be a multi-system approach and I think GPs, especially the bigger practices, can play a role,’ he said.
‘The smaller general practices may end up playing a role with the delivery of other COVID vaccines, which only require refrigeration or perhaps if they’re at -20°C. But most GPs have got refrigerators and will be able to distribute the kind of vaccine that doesn’t need freezing.’
 Victorian GP Dr Vyom Sharma says a real-world rollout of the vaccine will provide accurate expectations of what to expect in the next 12 months.
Victorian GP Dr Vyom Sharma says a real-world rollout of the vaccine will provide accurate expectations of what to expect in the next 12 months.
If the UK rollout runs smoothly, both Dr Sharma and Professor Booy believe it will give Australians greater confidence and ultimately have an influence on local uptake.
‘I can [understand] that some people are hesitant about a brand new vaccine. It will be interesting to see how significant a problem that is, and what we can do to counter any unwarranted concerns,’ Dr Sharma said.
‘Australian healthcare practitioners and experts will get an early read on this, and we can pre-empt any anxieties before they transform into scepticism that is weaponised by anti-vaxxer organisations.’
While Professor Booy says the prospect of one or more vaccine candidates being approved by January is an ‘exciting development’, he does urge caution.
‘We’ve made the kind of progress that we hoped we would. We’ve never had more great minds and we’ve never had more great technology applied on one problem – that’s why we’ve been able to be so fast,’ he said.
‘It’s exciting, but there’s no doubt we need to maintain caution. We’ve got our own TGA, which can make careful considerations around the important questions. And we also have access, very excitingly, to the data being developed in other countries and that’s being shared quite liberally from North America, from Europe and from the UK.
‘We can’t totally guarantee that there won’t be untoward problems. We’ve already seen with the AstraZeneca vaccine different results with different doses of vaccine and that needs to be clarified and understood.
‘We should be expecting hiccups, but we should also be excited by the progress we’re making.’
News of the vaccine being approved in the UK comes in the wake of the country recording more than 13,400 new cases of COVID-19 in a 24-hour period, taking the total number of recorded cases to more than 1.6 million and 59,000 deaths.
Australia’s Federal Health Minister Greg Hunt welcomed the vaccine’s emergency approval as ‘an important step for the world’.
‘I have again spoken to the Australian CEO of Pfizer. They remain on track for vaccine delivery once it is approved for use in Australia by the independent regulator,’ he said in a statement.
Pfizer is one of four COVID-19 vaccines for which the Australian Government has signed purchase agreements, having secured 10 million doses to be available from early 2021, pending regulatory approval.
Minister Hunt, however, did reiterate that Australia’s vaccine timeline remains unchanged.
‘Our advice remains that the timeline for a decision on approval is expected by the end of January 2021, and our planning is for first vaccine delivery in March 2021,’ he said.
‘Safety is our number priority and Australia is well placed both for a thorough, but rapid, safety assessment and early rollout of a free, voluntary but entirely universally available COVID-19 vaccine program.’
Log in below to join the conversation.
coronavirus COVID-19 mRNA pandemic vaccine
newsGP weekly poll
Within general practice, do you think there are barriers to providing flu vaccinations? If so, what are they?