News
GPs to be at forefront of Australia’s COVID vaccine distribution
The rollout could begin as early as March next year, following news the Federal Government secured two more potential candidates.
 Prime Minister Scott Morrison has announced Australia has secured 40 million doses of Novavax’s COVID-19 vaccine candidate.
Prime Minister Scott Morrison has announced Australia has secured 40 million doses of Novavax’s COVID-19 vaccine candidate.
The latest deals mean Australia has now secured 50 million additional doses of coronavirus vaccines currently in the final stages testing, bringing the overall total to 134 million.
On top of the previously announced commitments to manufacture 84 million vaccine doses in Australia, the Federal Government has announced it will import 40 million doses of Novavax’s candidate, along with another 10 million from Pfizer/BioNtech.
If successful, AAP has reported people will be able to initially access the vaccines from GPs, GP respiratory clinics, state and territory vaccination sites, and workplaces such as aged care facilities.
This approach would see GPs at the forefront of preparing the public and supporting vaccine uptake, in line with calls previously made by the RACGP.
Following the announcement, Federal Health Minister Greg Hunt predicted the first COVID-19 vaccine doses could be administered by as early as March next year.
‘We’re already at a massive advantage globally, but these vaccines will help give real protection right across the country,’ Minister Hunt told the Today show.
‘The most likely date for the health workers and the earliest vaccinations of elderly, if it’s approved for them, will be in March and then progressively we will roll out through the year.
‘But as soon as they [are] approved and safe and available, then we will make sure that they’re available for the entire Australian population.’
However, Minister Hunt later told Sunrise that the guidance is ‘not a guarantee’.
‘If they were available earlier they would be made available earlier, if it takes a little bit longer that would be the case,’ he said.
‘It is absolutely clear that we are on a path to having vaccines for all Australians during the course of 2021’.
The latest agreements mean Australia has lined up four different vaccine candidates, which Head of the Biosecurity Program at the Kirby Institute Professor Raina MacIntyre previously said is an important safeguard in case some fail to pass clinical trials.
‘If we focus only on one or two vaccines and they end up being non-starters or have serious side effects, we could be left out in the cold,’ she said.
‘It is best to choose several different vaccine technologies – mRNA-based, vectored, subunit etcetera – as each technology will have unique features and may also be associated with safety.’
Of the candidates now secured for use in Australia (pending safety approvals), AZD1222 uses a non-replicating viral vector platform, Pfizer/BioNtech is mRNA based, and the University of Queensland candidate utilises a unique molecular clamp stabilised spike protein with MF59 adjuvant. Novavax’s candidate is also protein-based.
According to Prime Minister Scott Morrison, manufacturing for Oxford University and AstraZeneca’s candidate will start in Melbourne next week, despite still being in the midst of phase 3 clinical trials that were previously halted due to safety concerns.
Department of Health secretary Professor Brendan Murphy has confirmed the first doses of AZD1222 vaccine will come from overseas, before being topped up by locally made supplies later in the year.
Although promising, the Australian boss of Johnson & Johnson’s pharmaceuticals business, Janssen, has warned plans for COVID-19 vaccine distribution remain a work in progress and will involve hurdles.
According to Nine Newspapers, Janssen ANZ managing director Bruce Goodwin said while Australia’s supply deals put the country in a good position, the mammoth task of distribution remains.
This follows a similar warning issued by University of Melbourne’s Dr Lusheng Shao, who recently said ‘the next big challenge’ is to transport and deliver any potential vaccine candidate safely and efficiently.
‘A successful immunisation program is built on a functional, end-to-end supply chain system,’ he said. ‘However, the vaccine supply chain is much more complex, even compared to other medical products such as masks and PPE [personal protective equipment].
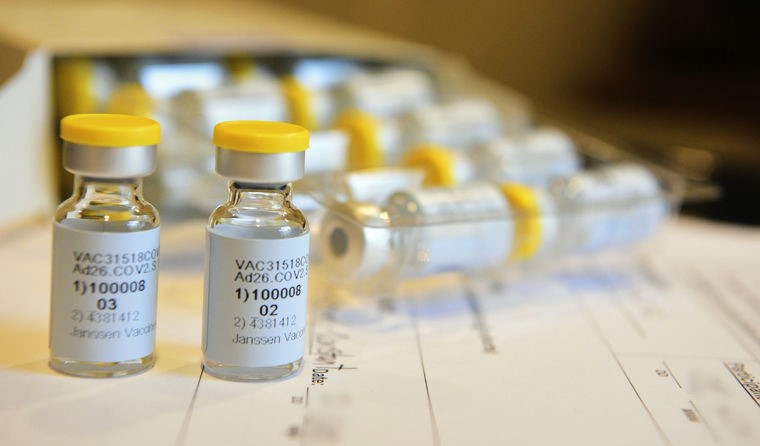 Johnson & Johnson has developed its own coronavirus vaccine candidate that is currently in phase 3 clinical trials.
Johnson & Johnson has developed its own coronavirus vaccine candidate that is currently in phase 3 clinical trials.
‘By its very nature, a vaccine is fragile and perishable. It must be kept in a sterile environment with strict temperature control, usually within 2–8⁰C. At least $25.9 million in Australian vaccines has been lost to cold-chain breaches between 2014 and 2019.
‘To minimise vaccine wastage, targeted education and training programs are necessary for healthcare workers.’
Cold-chain issues could be particularly pronounced for Pfizer/BioNTech’s candidate, which reportedly needs to be held in storage at -70°C and will last for only 24 hours at refrigerated temperatures between 2–8°C.
Pfizer has disputed this assertion and claims its vaccine can instead be stored at refrigerated temperatures for up to two days. But analysts have argued storage requirements mean it could only be used at certain hospitals or clinics with the proper equipment, and would require ‘intensive one-day vaccination events’ that would only cover ‘a fraction’ of the healthy population.
Dr Shao has also warned of issues associated with scaling up manufacturing capacity, as existing facilities are often purpose-built for specific vaccines. Additionally, there are concerns over whether there will be enough glass vials to store the vaccines or syringes to administer them.
‘While no shortages of these ancillary supplies have been reported in Australia, the upstream manufacturing industries rely heavily on imports,’ Dr Shao said.
‘Challenges also lie in domestic and global shipping, cold-chain transit, and distribution. There is limited airfreight capacity due to the border restrictions in most countries.
‘Another key challenge involves the delivery of vaccines, especially for remote and rural communities.’
The Novavax vaccine, made in the US and Czech Republic, will require two doses per person, with the first supply expected to arrive in early 2021.
The Pfizer–BioNTech vaccine candidate is also set to arrive in a similar timeframe and will be made in the US, Belgium and Germany.
None of the vaccines will be mandatory and all will be made available free of charge.
Log in below to join the conversation.
coronavirus COVID-19 vaccine
newsGP weekly poll
As a GP, do you use any resources or visit a healthcare professional to support your own mental health and wellbeing?