News
More than 40 children given incorrect dose of COVID-19 vaccine
No serious adverse effects were reported among the tiny proportion of children aged 5–11 who received an adult dose.
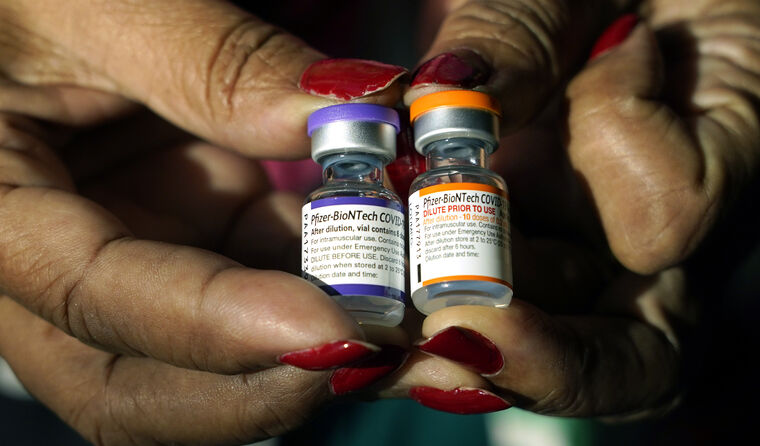 Vaccine doses for younger children come in different packaging to those intended for adults. Image: AAP Photos
Vaccine doses for younger children come in different packaging to those intended for adults. Image: AAP Photos
The latest Therapeutic Goods Administration (TGA) safety report has disclosed there have been 42 recorded instances of vaccination errors among 5–11-year-olds.
Of the reports, which represent a tiny fraction of the vaccine doses administered to younger children so far, there were four adverse events recorded with no serious outcomes, according to the TGA.
‘These were common and expected reactions including fever, headache and injection-site reactions,’ the report reads.
The TGA states that most of the errors were among the older end of the age group, when 10- or 11-year-olds received an adult dose of the Pfizer COVID-19 vaccine.
There were also ‘a few reports’ among younger children, according to the TGA, although the exact number is not specified.
The paediatric dose of the Pfizer is a third of the adult dose and contained in orange-capped vials, instead of grey or purple capped vials for those aged 12 and above.
Professor Robert Booy, an infectious diseases paediatrician and vaccine expert at the University of Sydney, said the errors should not be viewed with undue concern particularly among older children.
‘An 11-year-old is almost as big as a 12-year-old so I wouldn’t be too concerned. I wouldn’t be worried about long term side effects,’ Professor Booy told newsGP.
He said the most widely seen adverse reactions, including a sore arm, a fever, shivers, as well as enlarged lymph nodes in the neck and armpit could happen more often with a full dose among younger children.
‘You got more in the way of common side effects with a higher dose, but no greater immunogenicity,’ Professor Booy said.
‘The concern is that a higher dose may be more likely to give a hyper inflammatory condition with an increased risk of myocarditis as a side effect.
‘That’s the theoretical concern. With so few numbers, you’re not going to see it.’
In its report, the TGA highlighted the potential for age-related vaccine mistakes to take place and posted a reminder of the requirement for health professions to complete specific vaccination training.
It also suggests that parents ‘clearly communicate’ their child’s age when taking them to be vaccinated to ensure the right formulation is used.
‘Unlike other medicines, the … Pfizer vaccine dosage does not vary according to the child’s weight but depends on the age of the child on the day of vaccination,’ the reports says.
According to the latest COVID-19 government figures, 1,081,430 first doses have been administered to children within the age group, representing a first dose vaccination rate of around 47%.
The proportion of wrong doses recorded is approximately 0.0042% of the total given so far – or around four in every 100,000 doses.
The vast majority of the doses received so far have been first doses. The recommended gap between doses stands at eight weeks, with a shorter interval only recommended for children at greater risk.
At the time of publication, five weeks have passed since the vaccination program was opened to 5–11-year-olds last month.
The TGA report says 523 adverse reactions have been reported among the age cohort so far, with the most common including chest pain, vomiting, fainting, nausea and fever.
While there have been 10 reports of suspected myocarditis up until 6 February, the TGA said none were likely to represent myocarditis according to international criteria on further investigation.
The report states there has been one likely instance of pericarditis, a mild case in a 10-year-old boy.
The Pfizer vaccine is the only one approved for this age group. Moderna has submitted an application to allow its vaccine to be used among 6–11-year-olds and is awaiting a TGA decision.
A previous TGA safety report from last month suggests the early data shows vaccination among young children is safe, with fewer side effects recorded compared to older age groups.
The figures so far indicate the vaccination program of young children in Australia is following a similar pattern to that observed in the United States, where health authorities approved vaccine doses for 5–11-year-olds in November.
The TGA says reports of serious adverse effects following vaccination in children in the US were ‘extremely rare’ with 100 from 8.7 million vaccine doses delivered. There were 11 confirmed reports of myocarditis, all of which were mild.
Professor Booy also believes there has been no clear case yet established for vaccinating children younger than five.
‘We may not need to vaccinate under-fives if Omicron goes away,’ he said.
‘We certainly don’t have good evidence from the US yet to support safety and effectiveness, so we need to put that on the back burner.’
Log in below to join the conversation.
children’s vaccination COVID-19 vaccination myocarditis Pfizer
newsGP weekly poll
Should domestic and family violence training be mandatory for GPs?