Primary aldosteronism, also known as Conn’s syndrome, refers to inappropriately high and autonomous aldosterone production that is not suppressible by salt-loading.1 Aldosterone excess exerts negative feedback on renin production, leading to an increased aldosterone-to-renin ratio (ARR). Primary aldosteronism is the most common specifically treatable and potentially curable cause of hypertension.2 On the basis of predominantly international literature, primary aldosteronism accounts for 3.2–12.7% of hypertension in primary care and up to 30% in referral centres.3
There are limited data regarding the epidemiology and diagnosis of primary aldosteronism in Australia; its prevalence in the primary care setting is unclear because of the tendency for studies to focus on special subgroups or patients in tertiary referral centres.4 Two key studies of primary aldosteronism prevalence in Australia were conducted by the Endocrine Hypertension Unit led by Professor Michael Stowasser in Brisbane.1 One study, involving 199 normokalaemic patients with hypertension, reported the incidence of primary aldosteronism at 15%,5 while another study of 52 drug trial volunteers reported an elevated ARR in 12% of its participants.6
Despite a reported high prevalence, patients with hypertension are rarely screened for primary aldosteronism.7 This could be attributed to primary aldosteronism commonly presenting as normokalaemic hypertension, whereas hypokalaemia, traditionally considered a hallmark of primary aldosteronism, is present in only 20% of cases.1 The comprehensive Better the Evaluation and Care of Health (BEACH) program involving 16 years (April 2000 – March 2016) of data collection from general practitioners (GPs) revealed that aldosterone measurement was only ordered on 66 occasions in 1,568,100 GP–patient encounters (personal communication from Christopher Harrison). A survey of 500 GPs from Italy and Germany revealed that only 7% of patients with hypertension were screened for primary aldosteronism before commencement of non-specific antihypertensive medications.8 This is likely to be associated with the outdated teaching that primary aldosteronism is a rare and benign cause of hypertension, and/or the limited availability of screening and follow-up investigations for primary aldosteronism.9
An accurate and timely diagnosis of primary aldosteronism is crucial because it carries a much higher risk of adverse cardiovascular outcomes, such as stroke, myocardial infarction and atrial fibrillation, than blood pressure–matched essential hypertension.10 Aldosterone, acting via the mineralocorticoid receptor, has been shown to induce cardiovascular inflammation, fibrosis and remodelling.11 Unlike non-specific antihypertensive drugs, targeted management with adrenalectomy for unilateral primary aldosteronism or mineralocorticoid receptor antagonist treatment for bilateral primary aldosteronism not only normalises or improves blood pressure control, but also reverses or limits the additional cardiovascular injury caused by aldosterone excess.12
To improve the detection of primary aldosteronism, an understanding of its current pattern of diagnosis is important. This study, therefore, aims to analyse the referral pattern and disease characteristics of patients with hypertension referred to the Endocrine Hypertension Service (EHS) at Monash Health, the largest public health service in Victoria. The EHS, a referral service for patients with suspected primary aldosteronism, was established to meet the increasing demand of managing patients with primary aldosteronism13 and optimise their management. Gaps in the diagnosis of primary aldosteronism will be identified so that future strategies can be formulated for an earlier detection and targeted management.
Methods
We analysed the referral pattern and disease characteristics of 87 patients with hypertension who attended the EHS over a one-year period since its establishment in May 2016. The research study protocol was submitted to the Monash Health Human Research Ethics Committee and was exempted from review as it does not fall within the category of a research project within the National Statement on Ethical Conduct in Human Research (NHMRC, 2007). Data were collected prospectively as part of routine clinical practice. Each patient completed a questionnaire that covered sociodemographics, lifestyle patterns (smoking history, alcohol consumption and exercise), diagnosis of hypertension (year, reason and medications) and comorbidities. Sources of referral, clinical outcomes and biochemical outcomes were obtained from the medical records and Monash Pathology database.
At Monash Health, patients were switched from interfering antihypertensive medications (angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, dihydropyridine calcium channel blockers, beta-blockers) to non-interfering antihypertensive medications (verapamil, hydralazine, prazosin, moxonidine) four to six weeks before the screening ARR blood test. However, in a small number of cases where withdrawal of interfering agents was contraindicated because of previous aortic dissection, recent stroke or heart failure, ARR was done on existing medications and interpreted accordingly. Screening was considered positive when the ARR was >70 pmol/mU (where aldosterone was measured in pmol/L and direct renin concentration in mU/L) on the Diasorin Liaison assay on at least two independent samples. Plasma aldosterone of >140 pmol/L after a conventional recumbent saline suppression test, where 2 L saline is infused intravenously over four hours, confirmed the diagnosis of primary aldosteronism. Patients with confirmed primary aldosteronism then underwent a dedicated computed tomography (CT) scan of the adrenal glands followed by adrenal vein sampling to determine the subtype of primary aldosteronism. Patients with unilateral adrenal hyperplasia or unilateral aldosterone-producing adenoma were managed surgically with a unilateral adrenalectomy. Patients with bilateral adrenal hyperplasia and those who were unwilling or unable to undergo surgery were managed medically with a mineralocorticoid receptor antagonist (spironolactone as the primary agent or eplerenone as an alternative).
Analysis of outcomes was limited to 62 patients who had received targeted treatment for more than three months. It excludes patients who were awaiting investigations for primary aldosteronism subtyping, awaiting adrenalectomy or had not completed follow-up appointments at the EHS.
The Statistical Package for Social Science for Windows (version 24, SPSS, Chicago, Illinois, USA) was used to calculate mean, standard deviation, and P-value. Categorical variables were analysed by Pearson chi-squared test, while continuous variables with non-parametric distribution were analysed by Mann-Whitney U test. Statistical significance was set as P-value <0.05.
Results
Patient demographics and clinical characteristics
Table 1 shows the distribution of the study participants according to sex, mean age, mean number of antihypertensives, ethnicity, mean systolic blood pressure (BP), mean diastolic BP, body mass index (BMI), hip–waist ratio (HWR), potassium, aldosterone, renin and initial screening ARR. Among the 87 patients, 62 (71.3%) were diagnosed with primary aldosteronism, 12 (13.8%) did not have primary aldosteronism (ie non–primary aldosteronism group), and 13 (14.9%) were classified as ‘undefined’, meaning they were either unable to complete the formal investigations for primary aldosteronism (n = 7) or lost to follow-up (n = 6).
There was no statistically significant difference in gender distribution, mean number of antihypertensive medications, mean diastolic BP, BMI, and HWR between the primary aldosteronism and non–primary aldosteronism groups. Patients with primary aldosteronism had a significantly higher mean age of 54.6 years (95% confidence intervals [CI]: 51.4, 57.8) than patients without primary aldosteronism (mean age 44.3 years; 95%: CI 34.2, 54.4), significantly higher mean systolic BP of 132 mmHg (95% CI: 156, 167) compared with patients without primary aldosteronism (mean systolic BP 134 mmHg; 95% CI: 127, 142). As expected, patients with primary aldosteronism had a significantly lower potassium level of 3.9 mmol/L (95% CI: 3.7, 4.0), higher aldosterone level of 571.4 pmol/L (95% CI: 467.7, 675.0), significantly lower renin level of 4.7 mU/L (95% CI: 3.6, 5.9), and ultimately significantly higher initial screening ARR of 252 (95% CI: 179, 326), compared with patients without primary aldosteronism (Table 1).
|
Table 1. Comparison of characteristics of patients referred to the Endocrine Hypertension Service
|
|
Patient characteristics
|
Primary aldosteronism
(n = 62)
|
Not primary aldosteronism
(n = 12)
|
P
|
|
Males/females
|
32/30
|
6/6
|
0.919
|
|
Mean age (years)
|
54.6 (51.4, 57.8)
|
44.3 (34.2, 54.4)
|
0.039
|
|
Mean number of antihypertensive medications
|
2.6 (2.2, 3.0)
|
2.3 (1.4, 3.3)
|
0.772
|
|
Ethnicity
|
|
|
0.564
|
|
Oceanian
|
20 (32%)
|
7 (58%)
|
|
|
North West European
|
4 (8%)
|
0 (0%)
|
|
|
Southern & Eastern European
|
10 (16%)
|
1 (8%)
|
|
|
North African and Middle Eastern
|
6 (10%)
|
0 (0%)
|
|
|
South East Asian
|
4 (7%)
|
1 (8%)
|
|
|
North East Asian
|
4 (7%)
|
2 (17%)
|
|
|
Southern and Central Asia
|
10 (16%)
|
1 (8%)
|
|
|
People of the Americas
|
2 (2%)
|
0 (0%)
|
|
|
Sub-Saharan African
|
1 (2%)
|
0 (0%)
|
|
|
Mean systolic blood pressure (mmHg)
|
162 (156, 167)
|
134 (127, 142)
|
<0.0001
|
|
Mean diastolic blood pressure (mmHg)
|
98 (94, 101)
|
92 (83, 101)
|
0.280
|
|
BMI (kg/m2, normal 18.50–24.99)
|
30.7 (29.0, 32.4)
|
32.6 (27.6, 37.6)
|
0.679
|
|
Hip-to-waist ratio (normal <0.9 in males, <0.8 in females)
|
0.95 (0.93, 0.98)
|
0.98 (0.92, 1.03)
|
0.439
|
|
Potassium (mmol/L, normal 3.5–5.2)
|
3.9 (3.7, 4.0)
|
4.3 (4.1, 4.5)
|
0.015
|
|
Aldosterone (pmol/L, normal 70–1090)
|
571.4 (467.7, 675.0)
|
410.5 (261.3, 559.6)
|
0.073
|
|
Renin (mU/L, normal 4.4–46.0)
|
4.7 (3.6, 5.9)
|
66.5 (5.2, 127.9)
|
<0.0001
|
|
Initial screening ARR (normal <70)
|
252 (179, 326)
|
35 (1, 70)
|
<0.0001
|
|
All values are expressed as mean (95% confidence interval), except ethnicity, which is expressed as the number of participants of specified ethnicity (percentage of each relevant group)
ARR, aldosterone to renin ratio; BMI, body mass index
|
Duration of hypertension prior to referral to the EHS
The average duration of hypertension before referral to the EHS was 13.5 years. The data show that 71% of patients had hypertension for at least five years, 61% had hypertension for at least 10 years and 38% had hypertension for at least 15 years. Twenty per cent of patients had hypertension for one to two years (Figure 1).
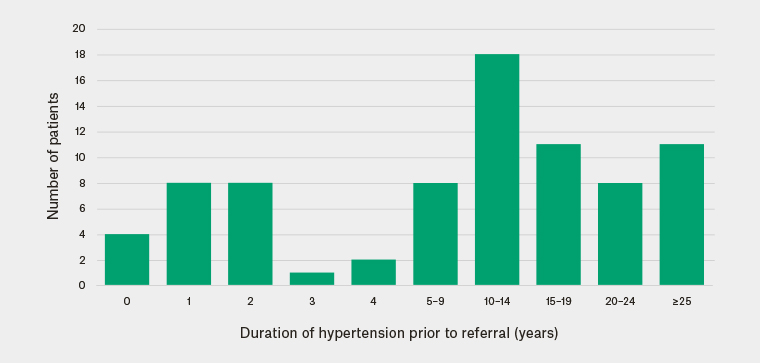
Figure 1. Duration of hypertension before referral to the Endocrine Hypertension Service
Current pattern of primary aldosteronism screening and referral to the EHS
The current screening recommendations for primary aldosteronism made by the Endocrine Society are shown in Box 1.9 On the basis of these guidelines, 69.2% of patients who were eventually referred to the EHS had sufficient indications to be screened for primary aldosteronism in the primary care setting. However, only 3.7% were actually screened for primary aldosteronism right from the outset, while the rest were initially referred to specialists for other reasons, with the most common reasons being hypertension (36.8%), an incidental finding of an adrenal lesion (17.5%), and hypokalemia (10.5%). It then took an average of 1.5 years before primary aldosteronism–specific investigations were conducted.
|
Box 1. Current screening recommendations for primary aldosteronism by the Endocrine Society9
|
-
Sustained blood pressure (BP) above 150/100 mmHg on each of three measurements obtained on different days, with hypertension (BP >140/90 mmHg) resistant to three conventional antihypertensive drugs (including a diuretic), or controlled BP
(<140/90 mmHg) on four or more antihypertensive drugs
-
Hypertension and spontaneous or diuretic-induced hypokalaemia
-
Hypertension and adrenal incidentaloma
-
Hypertension and sleep apnoea
-
Hypertension and a family history of early onset hypertension or cerebrovascular accident at a young age (<40 years)
-
All hypertensive first-degree relatives with primary aldosteronism
|
Only 3% of referrals to the EHS were made at first presentation of hypertension, while 29.5% were for hypertension with associated end-organ damage. The majority of referrals (77%) were derived from tertiary hospital departments, in particular, endocrinology (44%), nephrology (7%), and cardiology (7%), with only 23% from primary care.
Distribution of comorbidities and end‑organ damage
End-organ damage is defined as chronic renal failure (estimated glomerular filtration rate [eGFR] <60 mL/min/1.73m2), left ventricular hypertrophy (LVH; left ventricular mass index >115 g/m2 in males or >95 g/m2 in females), stroke or atrial fibrillation. Of the patients with confirmed primary aldosteronism, 26/62 (42%) already had end-organ damage on presentation to the EHS in contrast to only one of the 12 patients without primary aldosteronism (8%). The most common comorbidities in patients with primary aldosteronism were dyslipidaemia (42%), obesity (37%), diabetes mellitus (31%), LVH (18%) and obstructive sleep apnoea (OSA; 18%; Figure 2). However, the prevalence of LVH might be underestimated, as only 27 patients with primary aldosteronism had an echocardiogram, of whom 31% had LVH and 4% had left ventricular failure (ejection fraction <50%).
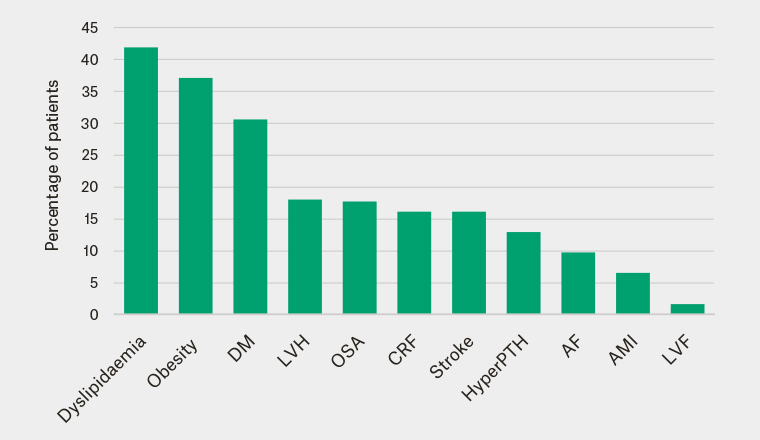
Figure 2. Distribution of baseline comorbidities in patients with primary aldosteronism
Obesity, BMI ≥30; DM, diabetes mellitus; LVH, left ventricular hypertrophy, left ventricular mass index >115g/m2 in males or >95g/m2 in females; OSA, obstructive sleep apnoea; CRF, chronic renal failure, eGFR <60; HyperPTH, Hyperparathyroidism; AF, atrial fibrillation; AMI, acute myocardial Infarction; LVF, left ventricular failure, ejection fraction <50%.
Outcomes of targeted management in patients diagnosed with primary aldosteronism
Of the 62 patients with primary aldosteronism, 14 were surgically managed, 37 were medically managed and 11 were still awaiting subtyping or surgery. Targeted management of primary aldosteronism resulted in improved clinical and biochemical outcomes.
On the basis of the standardised outcome criteria after adrenalectomy as outlined in the Primary Aldosteronism Surgical Outcome study,14 complete clinical success was achieved in 29% (n = 4) of the surgical patients and partial clinical success in 71% (n = 10); complete biochemical success was seen in 93% (n = 13), and partial biochemical success was seen in 7% (n = 1). All the patients who underwent adrenalectomy had a statistically significant decrease in BP; the mean systolic BP decreased from 162 mmHg to 132 mmHg, while the mean diastolic BP decreased from 100 mmHg to 91 mmHg. Ninety-three per cent of patients who underwent adrenalectomy required significantly fewer antihypertensive medications, with the mean number of antihypertensive medications decreasing from 2.2 pre‑adrenalectomy to 0.8 post‑adrenalectomy. All of the patients who underwent adrenalectomy had an increase in potassium; the the mean potassium level increased from 3.5 mmol/L to 4.7 mmol/L. The mean renin level increased from 3.04 mU/L to 18.98 mU/L (normal: 4.4–46.0 mU/L) and the mean ARR decreased from 553 to 31 (normal: <70). These biochemical changes were all statistically significant (Table 2).
In the absence of an international consensus on outcomes of medical treatment, we defined complete clinical success as normalised blood pressure (systolic BP <140 mmHg and diastolic BP <90 mmHg) on just a mineralocorticoid receptor antagonist (either spironolactone or eplerenone). Partial clinical success was defined as same blood pressure as before the primary aldosteronism diagnosis, with fewer antihypertensive medications, or reduced blood pressure with the same number of or fewer antihypertensive medications. Biochemical success was defined as normalisation of renin and/or correction of hypokalaemia when applicable. Complete clinical success was achieved in 35% (n = 13) and partial clinical success in 65% (n = 24) of patients who received medical management; biochemical success was achieved in 100% of these patients (n = 37). All the patients who received medical management had a statistically significant decrease in BP; the mean systolic BP decreased from 167 mmHg to 131 mmHg, and the mean diastolic BP decreased from 99 mmHg to 84 mmHg. Seventy-eight per cent of patients who received medical management required significantly fewer antihypertensive medications, with the mean number of antihypertensive medications decreasing from 3.0 pre‑medical management to 2.1 (including a mineralocorticoid receptor antagonist) post–medical management. All patients who received medical management had significant biochemical success, with an increase in mean renin level from 4.50 mU/L to 43.69 mU/L and an increase in mean potassium level from 3.9 mmol/L to 4.6 mmol/L (Table 2).
|
Table 2. Outcomes of targeted management in patients diagnosed with primary aldosteronism
|
|
|
Adrenalectomy (n = 14)
|
Medical Management (n = 37)
|
|
Pre
|
Post
|
P*
|
Pre
|
Post
|
P†
|
|
Clinical outcome
|
Mean systolic blood pressure (mmHg)
|
162
|
132
|
<0.0001
|
167
|
131
|
<0.0001
|
|
Mean diastolic blood pressure (mmHg)
|
100
|
91
|
0.038
|
99
|
84
|
<0.0001
|
|
Mean number of antihypertensive medications
|
2.2
|
0.8
|
0.002
|
3.0
|
2.1
|
0.016
|
|
Range of slow-K per day
|
0–13
|
0
|
0.007
|
0–6
|
0
|
0.022
|
|
Biochemical outcome
|
Potassium (mmol/L)
|
3.5
|
4.7
|
<0.0001
|
3.9
|
4.6
|
<0.0001
|
|
Renin (mU/L)
|
3.04
|
18.98
|
<0.0001
|
4.50
|
43.69
|
<0.0001
|
|
ARR
|
553
|
31
|
<0.0001
|
214
|
NA
|
NA
|
|
*Comparing clinical and biochemical data pre-adrenalectomy to post-adrenalectomy.
†Comparing clinical and biochemical data pre-medical management to post-medical management.
ARR, aldosterone to renin ratio.
|
Discussion
In our limited sample, we found that the diagnosis of primary aldosteronism in Victoria was delayed, with the majority of patients investigated and diagnosed after a prolonged period of hypertension, often with associated end-organ damage. Despite 70% of these patients meeting the Endocrine Society recommendations for primary aldosteronism screening at the time of their initial referral to a specialist unit, only 3% were screened from the outset, while the remainder waited another 1.5 years for the appropriate investigations to take place. The lack of awareness of international guidelines for primary aldosteronism screening is consistent with the findings by Mulatero et al in Italy and Germany.8 The number of EHS referrals from GPs (23%) in this study is most likely to be an overestimation of referrals to investigate primary aldosteronism by the primary care sector, as the EHS had actively delivered education sessions to local GPs. Therefore, GPs in the catchment of Monash Health would have had an increased awareness of primary aldosteronism and may have screened more patients than GPs in other regions.
In this study, more than half of the patients had hypertension for more than 10 years before being referred for specialist review. With a delayed diagnosis of primary aldosteronism, a greater number of non-specific antihypertensive medications is required; 55% of patients took three or more antihypertensive medications at the time of referral. This in turn complicates the evaluation process because of the confounding effects of multiple commonly used antihypertensives on aldosterone and renin levels.9,12 A washout of all interfering antihypertensive medications is potentially difficult in patients with severe hypertension, and a long transition process may delay or even prevent the diagnosis of primary aldosteronism.
Given the longstanding duration of hypertension and the known detrimental effects of aldosterone excess, it is not surprising that 42% of patients with primary aldosteronism already had end‑organ damage on presentation. The rate of end-organ damage recorded in our study is likely to be an underestimate because, first, half of the patients did not undergo an echocardiogram and therefore LVH could have been missed. Second, the study participants did not have routine CT of the brain to detect silent cerebrovascular ischaemic changes. Third, glomerular hyperfiltration mediated by aldosterone resulting in an increased eGFR may have masked underlying renal impairment in patients with primary aldosteronism. End-organ damage was less prevalent in the non–primary aldosteronism group, but statistical comparison cannot be carried out because of the small number of these patients. However, as reported in a meta-analysis of observational studies comparing 3838 patients with primary aldosteronism and 9284 patients with essential hypertension, patients with primary aldosteronism had an increased risk of stroke, coronary artery disease, atrial fibrillation, heart failure, diabetes mellitus, metabolic syndrome and LVH when compared with those with BP-matched essential hypertension.15 A timely diagnosis of primary aldosteronism and prompt treatment may have prevented the development of these complications.
Among the patients with primary aldosteronism, the most common comorbidities were dyslipidaemia (42%), obesity (37%) and diabetes mellitus (31%). It is important to beware of primary aldosteronism masquerading as a component of the metabolic syndrome. Fallo et al reported a significantly higher prevalence of metabolic syndrome among patients with primary aldosteronism (41.1%) than those with essential hypertension (29.6%).16 Aldosterone levels and BMI are positively correlated in patients with hypertension,17 and diabetes mellitus is more prevalent in patients with primary aldosteronism than those with essential hypertension.16 Another common comorbidity is OSA. One study reported a 79% prevalence of OSA among patients with primary aldosteronism and found that targeted primary aldosteronism treatment improved sleep parameters.18 In our study, OSA affected only 17% of patients with primary aldosteronism, which is probably an underestimate; the true prevalence can only be assessed by the routine use of sleep studies.
BP control improved in all the patients with primary aldosteronism who received targeted treatment for longer than three months, with 45% stopping all non-specific antihypertensive medications and 35% reducing their medications. The lack of complete BP normalisation is most probably due to prolonged exposure to aldosterone excess, which induces endothelial dysfunction and vascular remodelling,19,20 enhancement of sympathetic outflow20,21 and impairment of baroreceptor reflex function.22 Targeted management of primary aldosteronism minimises the excess risk of cardiovascular and cerebrovascular morbidity and mortality.23,24 As the degree of benefit achieved from targeted management of primary aldosteronism is inversely related to the duration of hypertension,25 an early diagnosis of primary aldosteronism at a younger age is most beneficial.26
The main limitation of this study is the relatively small number of patients evaluated in one tertiary centre. However, the EHS is the only centre dedicated to the management of primary aldosteronism in Victoria and captures the majority of patients from the large catchment area of Monash Health (>1.05 million patients). The finding that only 60 cases of primary aldosteronism were diagnosed over a year, within this vast catchment, supports the growing view that primary aldosteronism remains an under-diagnosed disease and therefore an under-treated cause of cardiovascular complications.7 The differences in the presentation of hypertension and associated complications between patients with and without primary aldosteronism in our study could be better substantiated by a larger number of matched patients with essential hypertension; however, our findings are consistent with previous meta-analyses, which also showed increased end-organ damage in patients with primary aldosteronism.15 Despite the size limitation, this study still presents a contemporary picture of primary aldosteronism diagnosis in the largest public health service in Victoria, highlights the clinically important issue of complex hypertension caused by primary aldosteronism, and argues that the screening guidelines for primary aldosteronism need increased recognition in both primary and tertiary health sectors to improve the detection of this modifiable cause of hypertension.
Conclusion
The current diagnosis of primary aldosteronism is suboptimal – its delayed diagnosis results in end-organ damage that requires complex management and complicates the evaluation process of primary aldosteronism. Given that appropriate management of primary aldosteronism produced significant clinical and biochemical improvement, an increased awareness of primary aldosteronism is required in both primary and tertiary care so that an earlier diagnosis can be made for optimal patient outcomes.
Implications for general practice
-
The rate of primary aldosteronism screening in primary care is low relative to the number of patients with hypertension who fulfil the current Endocrine Society screening recommendations.
-
Delayed diagnosis of primary aldosteronism after a prolonged period of hypertension is associated with increased end-organ damage.
-
Targeted management of primary aldosteronism leads to clinical and biochemical improvement in all patients.
-
An increased awareness of primary aldosteronism in both primary and tertiary care is needed to achieve an earlier diagnosis for optimal patient outcomes.