Osteoporosis is a common disorder of bone metabolism that affects an ageing population.1 Progressive microarchitectural deterioration and reduction in bone mineral density result in increased susceptibility to fragility fractures.2 Osteoporosis results in significant morbidity and mortality, with 50% of patients becoming permanently disabled and mortality rates as high as 20% within 12 months after a hip fracture.2,3 Despite strong evidence for efficacious treatment options, osteoporosis is an ongoing source of financial burden on healthcare systems, with each hip fracture costing the healthcare system $21,285 in the first year after hospitalisation. In Australia, estimated yearly costs directly associated with osteoporosis (population 24 million) are as high as $1.9 billion.4,5 Patients with cognitive impairment are 2.7 times more likely to have a hip fracture when compared with their age-matched and sex-matched counterparts without dementia.6 A population-based study of 2610 patients aged ≥66 years indicated that 25% of patients with dementia had at least one osteoporotic fracture over a four-year period, compared with 7% of their counterparts without dementia.7
Following a fracture, patients with dementia are less likely to recover to their pre-fracture functional status. Because of factors such as a high rate of falls, vitamin D deficiency, demographics and lifestyle,8 patients with dementia continue to be at a higher risk of adverse outcomes, including sustaining further fractures,9 functional decline, institutionalisation and mortality, compared with patients without dementia.10 In some studies, only dementia and age were independent predictors of mortality following a hip fracture.11,12
Despite an increase in morbidity due to osteoporotic fractures, which can lead to institutionalisation, emergency admissions, physician visits and an increased inpatient length of stay (LOS), osteoporosis remains underdiagnosed13,14 and undertreated,15–17 particularly among patients with dementia, compared with those without dementia.7,13,18 Under-treatment of osteoporosis in the elderly19,20 contributes to significant healthcare cost burden and is predicted to rise with our ageing population.
This study compares the rate of osteoporosis treatment in older patients with and without dementia presenting with a minimal trauma fracture (MTF) to a geriatrician-led orthogeriatric unit (OGU); secondary outcomes assessed were discharge outcomes and mortality outcomes at 30 and 90 days.
Methods
A prospective study of consecutive patients aged ≥65 years was undertaken over a period of 15 months. This study was approved by the institutional Human Research Ethics Committee as a Quality Assurance Project (quality improvement number 2420). Patients aged ≥65 years who were admitted to an acute OGU at a tertiary teaching hospital for management of MTF were included in the study. Patients <65 years of age and patients with non-osteoporotic pathological fractures were excluded. All patients were under the combined care of an orthopaedic and geriatric medical team. Information was confirmed from interviews with the patient and carer (where appropriate), and review of hospital, general practice, pharmacy, radiology and medical records as required. All patients included in this study were managed by a single geriatric team, and all assessments and data collection were performed by a single assessor (NM, geriatric medicine trainee).
Demographics and clinical outcome parameters were recorded and included osteoporosis and dementia history, treatment, type of MTF (neck of femur [NOF] or non-NOF), operative versus conservative management, number and type of comorbidities, common postoperative complications, LOS, residential status and discharge destination. Patients were categorised on the basis of prior history of osteoporosis and dementia. These diagnoses were verified by the clinicians involved in providing care. Prior osteoporosis was defined by a clinical history of osteoporosis (bone mineral density [BMD] T-score of –2.5 or less) or MTF or osteoporosis treatment. Dementia diagnosis was verified by the geriatric team on the basis of a combination of clinical assessment on admission, past medical history and/or active treatment. All patients had an Abbreviated Mental Test Score (AMTS) as a cognitive screening tool. AMTS is scored out of 10: a score of ≥7 indicates good cognitive function, 4–6 indicates mild to moderate dementia and <3 is consistent with advanced dementia.21
For statistical analysis, the study cohort was stratified into four groups: those with a history of prior osteoporosis, with and without dementia diagnosis; and those with no prior osteoporosis diagnosis, with and without dementia. Data were analysed using Statistical Package for the Social Sciences, version 18 (SPSS, INC, Chicago IL).
Patients’ characteristics were described using prevalence for categorical variables, and mean and standard deviation for continuous variables. A chi-square test was used to determine the pre-fracture and post-fracture osteoporosis treatment rate. Fisher’s exact chi-square was used to compare admission versus discharge treatment stratified by dementia status.
Results
The study cohort constituted 502 patients; 318 of these patients had a NOF fracture. The mean age of the study cohort was 82 years (± 9.53); 77% were >75 years of age. At the time of admission, 76% (n = 381) were living at home, either independently, with support provided by their families or formal community services. The remaining 24% (n = 121) were from either low-level care or nursing homes (Table 1).
| Table 1. Baseline demographic profile for osteoporosis and dementia status |
| Study cohort |
Total (n = 502)
Dementia (n = 226)
Prior osteoporosis
(n = 281) |
No prior osteoporosis
(n = 221) |
Prior osteoporosis
(n = 281) |
| No dementia |
Dementia |
No dementia |
Dementia |
|
Study cohort
n (%)
|
Total (n = 502) |
151 (30%) |
70 (14%) |
125 (25%) |
156 (31%) |
Gender
n (%) |
Female (n = 382) |
96 (25%) |
52 (14%) |
108 (28%) |
126 (33%) |
| Male (n = 120) |
55 (46%) |
18 (15%) |
17 (14%) |
30 (25%) |
Age
Mean (SD) |
Female (n = 382) |
78 (9.7) |
84 (9.1) |
81 (9.2) |
87 (7.7) |
| Male (n = 120) |
77 (8.3) |
77 (8.3) |
78 (11.1) |
86 (7.7) |
Age
n (%) |
≤75 (n = 113) |
55 (49%) |
11 (10%) |
35 (31%) |
12 (11%) |
| >75 (n = 389) |
96 (25%) |
59 (15%) |
90 (23%) |
144 (37%) |
| Previous fracture |
Total (n = 271) |
1 (0%) |
7 (3%) |
116 (43%) |
147 (54%) |
Admission MTF
(n = 502) |
Non-NOF (n = 184) |
68 (37%) |
10 (5%) |
55 (30%) |
51 (28%) |
| NOF (n = 318) |
84 (26%) |
59 (19%) |
70 (22%) |
105 (33%) |
| AMTS score |
Mean (range) |
8.3 (6–10) |
4.2 (0–7) |
8.02 (6–10) |
3.6 (0–8) |
| AChEI therapy |
On AChEI (n = 18) |
0 (0%) |
6 (33%) |
0 (0%) |
12 (67%) |
| No osteoporosis treatment |
133 (88%) |
58 (83%) |
41 (33%) |
43 (28%) |
| P = 0.29 |
P = 0.361 |
| Calcium |
11 (7%) |
4 (6%) |
51 (41%) |
75 (48%) |
| P = 0.78 |
P = 0.230 |
| Vitamin D |
13 (9%) |
10 (14%) |
64 (51%) |
109 (70%) |
| P = 0.24 |
P = 0.002* |
| ART |
2 (1%) |
1 (1%) |
41 (33%) |
33 (21%) |
| P = 1.00 |
P = 0.030* |
Combination osteoporosis treatment
(calcium + vitamin D + ART) |
1 (1%) |
0 (0%) |
20 (16%) |
25 (16%) |
| P = 1.00 |
P = 1.00 |
AChEI, acetylcholinesterase inhibitor; AMTS, Abbreviated Mental Test Score; ART, antiresorptive therapy; MTF, minimal trauma fracture; NOF, neck of femur; SD, standard deviation
*The Chi-square statistic is significant at the 0.05 level. |
Of the 502 patients, 226 (45%) had a dementia diagnosis with a mean AMTS score of 3.8 (± 2.25), and 281 (56%) had a prior diagnosis of osteoporosis on admission. A total of 156 (31%) had comorbid dementia and osteoporosis. Patients with dementia were more likely to have a prior diagnosis of osteoporosis than those without (56% versus 44%, P <0.001). The majority of cases (63%) admitted to the OGU with MTF had presented with a NOF fracture. Two-thirds (105/156) of those with comorbid dementia and osteoporosis pre-admission had presented with a NOF fracture. Overall, 72% (164/226) of those with a dementia diagnosis versus 56% (154/276) of those without a diagnosis of dementia presented with a hip fracture.
Approximately half of the cohort (238/502) had two or three comorbidities on admission. Common comorbidities included cardiovascular, respiratory, rheumatological and neurological disorders. Patients with dementia and prior osteoporosis history had a greater number of falls in the 12 months prior to admission. The main risk factors for falls were polypharmacy, osteoarthritis and neurological disorders. Average LOS was 20.26 (± 17.61) days.
Osteoporosis treatment prior to admission
Overall, rates of osteoporosis treatment were low in those admitted with an MTF to the OGU. Only 28% of the total study population were on calcium supplements and 39% were on vitamin D supplements at the time of admission. Rates of other osteoporosis-specific medications (ie antiresorptive therapy [ART]) were even lower at 15%. The most commonly used osteoporosis medications were bisphosphonates (12%). The use of combination therapy, such as calcium, vitamin D and ART, was low at 9%. Table 1 shows the distribution of the patients’ osteoporosis treatment on admission. Of those with established osteoporosis, 29% (84/281) were on no intervention at all on admission (ie no calcium, vitamin D or ART).
At admission, patients with comorbid osteoporosis and dementia were less likely to be on ART when compared with patients with osteoporosis without dementia (21% versus 33%; P <0.05). However, patients with comorbid osteoporosis and dementia were more likely to be on vitamin D, compared with those without dementia (70% versus 51%; P <0.005).
A greater number of patients with a prior history of osteoporosis were on calcium (45% versus 7%; P <0.001) and vitamin D supplements (62% versus 7%; P <0.001), compared with those without osteoporosis. In those with a diagnosis of osteoporosis, there was a higher rate of use of ART (26% versus 1%; P <0.001) and combination therapy (16.0% versus 0.5%; P <0.001), compared with those without osteoporosis.
Osteoporosis on discharge
Overall rates of osteoporosis treatment improved significantly on discharge from the OGU. At discharge, 81% of the total study population were on calcium supplements, compared with 28% at admission, and 90% were on vitamin D supplements, compared with 39% on admission from admission (P <0.01). Rates of ART improved from 16% to 43%, and rates of ART combined with calcium and vitamin D therapy increased from 9% to 39% (P <0.01). Table 2 summarises the improvement in treatment rate across subgroups by osteoporosis and dementia category, compared with baseline.
| Table 2. Osteoporosis treatment at discharge |
| Treatment status |
No osteoporosis (n = 221) |
Osteoporosis (n = 281) |
| Dementia status |
Dementia status |
No dementia
(n = 151) |
Dementia
(n = 70) |
P value |
No dementia
(n = 125) |
Dementia
(n = 156) |
P value |
| No treatment |
19 (13%) |
10 (14%) |
P = 0.831 |
2 (2%) |
8 (5%) |
P = 0.193 |
| Calcium |
117 (77%) |
49 (70%) |
P = 0.245 |
117 (94%) |
125 (80%)* |
P = 0.002 |
| Vitamin D |
127 (84%) |
59 (84%) |
P = 1.000 |
122 (98%) |
143 (92%)* |
P = 0.002 |
| ART |
50 (33%) |
17 (24%) |
P = 0.210 |
74 (59%) |
56 (36%)* |
P = 0.000 |
| Combination |
44 (29%) |
16 (23%) |
P = 0.417 |
70 (56%) |
50 (32%)* |
P = 0.000 |
| * The chi-square statistic is significant at the 0.05 level |
Patients with a diagnosis of established osteoporosis prior to admission were more likely to receive treatments for osteoporosis on discharge. However, treatment rates were higher in those without dementia, compared with those with dementia, when considering calcium (80% versus 94%) and vitamin D (92% versus 98%), with P <0.01 for both treatments. Patients with a diagnosis of established osteoporosis prior to admission who also had dementia were less likely to receive specific treatment such as ART (36% versus 59%; P <0.01) or ART combined with calcium and vitamin D (32% versus 56%, P <0.01).
Discharge outcomes and mortality
Although the total cohort of patients admitted with a fracture shifted to a greater level of need for care on discharge, patients with dementia were more likely to be admitted from residential care or home-based supportive care. Mortality rates were higher at 30 and 90 days in patients with osteoporosis who also had dementia, compared with those without a diagnosis of dementia (1.6% versus 6.4% at 30 days; 9.6% versus 17.3% at 90 days), equating to a doubling of the mortality risk as early as 90 days (Table 3; Figure 1).
| Table 3. Discharge and mortality outcomes in patients with and without dementia |
| Residential status |
Osteoporosis no dementia (n = 125) |
Osteoporosis with dementia
(n = 156) |
| At admission |
At discharge |
At admission |
At discharge |
| Home |
77 (61.6%) |
18 (14.4%) |
8 (5.1%) |
1 (0.6%) |
| Home with family support |
17 (13.6%) |
30 (24.0%) |
29 (18.6%) |
6 (3.8%) |
| Home with community support |
26 (20.8%) |
28 (22.4%) |
36 (23.1%) |
23 (14.7%) |
| Hostel |
4 (3.2%) |
2 (1.6%) |
22 (14.1%) |
12 (7.7%) |
| Nursing home |
1 (0.8%) |
3 (2.4%) |
61 (39.1%) |
59 (37.8%) |
| Transitional care program |
|
9 (7.2%) |
|
10 (6.4%) |
| Other hospital |
|
21 (16.8%) |
|
8 (5.1%) |
| 30-day mortality |
|
2 (1.6%) |
|
10 (6.4%) |
| 90-day mortality |
|
12 (9.6%) |
|
27 (17.3%) |
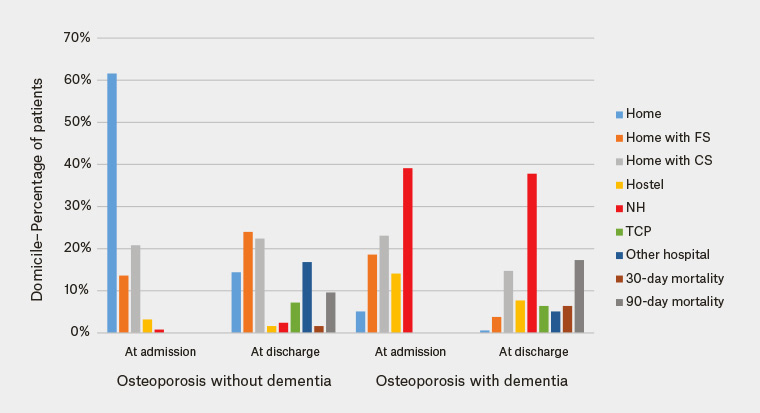
Figure 1. Discharge and mortality outcomes in patients with and without dementia
CS, Community Care Support Program; FS, family support; NH, nursing home; TCP, Transitional Care Program
Discussion
This single-centre study in Australia concurs with the international published literature that the two most important risk factors for mortality in this cohort are age and a diagnosis of dementia. These results concur with Gleason et al in extrapolating that patients with dementia are more likely than those without dementia to have known osteoporosis prior to fracture (43.8%, compared with 37.7%, P <0.05).13 On admission to the OGU, more than half (56%) of patients presenting with MTF had known osteoporosis, and 55% of those with osteoporosis had a prior diagnosis of dementia.
In this older, frailer cohort, as expected, patients were less likely to have been assessed for, or offered, preventive strategies that are available for primary and secondary prevention. Treatment rates on admission in the study cohort were low for all modalities of treatment including calcium (28%), vitamin D (39%) and ART (15%). Under-treatment of patients with both osteoporosis and dementia was significantly higher that that of patients with osteoporosis alone for all classes of osteoporosis treatment.
Our study cohort represents a population at an increased risk of further fragility fractures because of their advanced age, frailty, high prevalence of dementia, increased number of falls and previous history of MTF. The study results showed that those with prior osteoporosis and dementia were less likely to be on ART alone or ART combined with calcium and vitamin D supplements when compared with the non-dementia cohort. Our study confirms that these patients are more likely to present with a hip fracture (two-thirds) and are at higher risk of early mortality (90-day mortality close to the 12-month expected mortality in the general population following a hip fracture). Despite the high prevalence and potentially devastating impact of osteoporosis in patients with dementia, under-treatment has been extensively reported in numerous studies.7
Our results are consistent with previous studies in showing that patients with osteoporosis and dementia are more likely to be on calcium and vitamin D supplements, but less likely to be on ART when compared with their counterparts without dementia.7,13,22 Underprescribing may be due to concerns about compliance with oral therapy, as well as estimated shorter life expectancy for patients with dementia. Use of calcium and vitamin D supplements may also have been influenced by the uncertainty around the cardiovascular risk and osteoporosis benefit of these supplements. Initiation of ART with simpler dosing and shorter time to onset, or parenteral alternatives, could be beneficial in this patient subset. Examples of these include enteric-coated preparations that remove the need to fast, subcutaneous denosumab or annual zoledronic acid infusions.23
In our study, higher 90-day mortality occurred in patients with dementia who were, by definition, frailer, such as those who were older and had a history of hip fracture, prior osteoporosis and poor functional status compared with those who survived. Therefore, dementia appears to be a marker of frailty and osteoporotic fracture risk. These patients were the frailest population at high risk of all-cause mortality; therefore, mortality may have not been prevented at the advanced stages. These findings are consistent with the published findings that age, dementia and frailty are the most important factors predicting mortality after fracture admission.
This study shows that patients with osteoporosis and dementia are at a higher risk of morbidity and mortality, increasing dependence and higher care. It may be too late to consider intervention at this stage in the majority, and the goal should be for primary or secondary prevention prior to admission with fracture to prevent this presentation and the healthcare burden associated with this admission.
The strength of this study is that it was a prospective study with a quality assurance design that included a large cohort, and a single ‘observer’ collecting all included data. All patients aged ≥65 years who were admitted were included in the study. A comprehensive set of patient data was collected and collated prospectively, followed by a robust analysis and statistical validation of results.
A potential limitation of this study is that osteoporosis and dementia were defined on the basis of patients’ history, case notes and clinical assessment. This is subject to reporter bias. In addition, in clinical practice it is not always possible to verify the accuracy of previous documentation. However, this was compensated for by using multiple information sources and assessment by a geriatrician-led team to verify these diagnoses, which is arguably the gold standard.
This study is based on the audit of a single orthogeriatric unit. We were unable to determine the reasons why these patients were not ‘managed’ in terms of osteoporosis risk. Pre-existing frailty, comorbidity and patient choice may have been factors. There may be similar reasons for the difference in management of the two groups on the OGU. Furthermore, it may not reflect the standard of care in other similar settings and orthogeriatric care in terms of community-based preventive management and OGU intervention, which may account for the high morbidity. However, these findings are consistent with the published literature, which suggests this study does reflect existing practice that warrants improvement.
Another limitation of this study was that a number of patients were transferred to external hospitals with limited follow-up opportunity during the duration of the study. Further update of patient data could further enhance study outcomes.
Conclusion
This study shows a setting in which there is a low rate of treatment of older, frailer patients who are at a high risk of fracture, including those with demonstrated osteoporosis, prior fracture, dementia and falls risk. It would be useful to audit this in other similar Australian settings. It is reassuring that in a geriatrician-led OGU, overall osteoporosis treatment rate improved significantly post-MTF. However, in this cohort it may be a case of ‘too little, too late’, and it may be more realistic to aim for prevention rather than a cure. Pre-emptive intervention with known effective therapies in these high-risk patients prior to presentation with a fracture may have prevented at least some of these unnecessary, painful and costly admissions.
Implications for general practice
- Older patients with dementia are at a higher risk of osteoporosis and fractures.
- The imperative to assess and manage osteoporosis risk is significant.
- Untreated osteoporosis results in fracture, with high morbidity and mortality and costs.
- GPs are best positioned to assess, manage and reduce the risk in this high-risk population.