Abnormalities of testicular descent are very common in boys.1 However, confusion around terminology and current recommendations can prevent appropriate management.
Definitions
Maldescended testis
This term refers to any abnormality in testicular descent that is not a normal variant, and includes the conditions defined below.
Undescended testis
Undescended testis (UDT) is the second most common paediatric surgical condition after inguinal hernias.1 It refers to a testis that is not in the scrotum by the age of three months because of a failure of normal descent.1 Five per cent of boys have a UDT at birth, 1–2% at three months and 1% at one year; hence, it is uncommon for testes to descend after three months.1–3
Ectopic testis
An ectopic testis has deviated from the normal path of testicular descent (from the abdomen through the inguinal canal and into the scrotum) and may be found in almost any other region.
Ascending testis
An ascending or acquired testis may have descended previously but then moved to a higher position over time because of an abnormally persisting fibrotic remnant of the processus vaginalis (an outpouching of peritoneum that follows the testis as it descends into the scrotum from the abdomen). The remnant usually regresses in the first few years but can continue causing testicular ascent.3 Regular re-examination is recommended in a primary care setting even if the testes were previously noted in the scrotum (Figure 1).
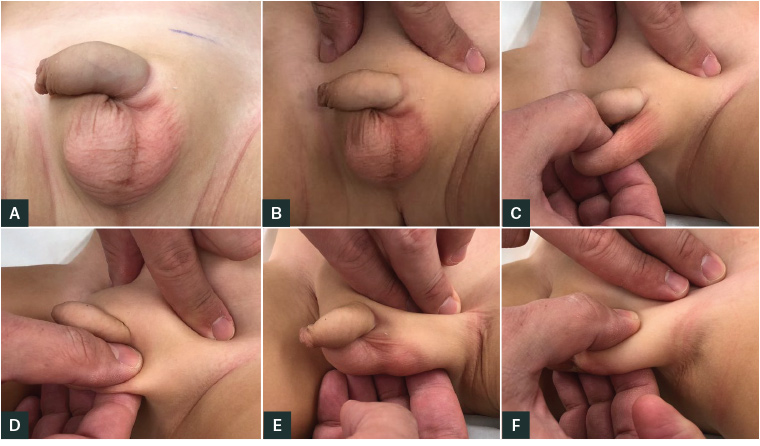
Figure 1. Clinical examination for a possible undescended testicle
A. Inspect the scrotum, note any scrotal hypoplasia and whether it appears. B. Occlude the external inguinal rings with digital pressure as this prevents retraction of the testicles with a cremasteric response. C. Palpate the normal-in-appearance hemi-scrotum. D. Palpate the hypoplastic hemi‑scrotum. E. Milk from the external ring to the scrotum to attempt to palpate the testicle.
F. Once located, assess whether the testicle can be moved to the scrotum.
Impalpable testis
Thirty per cent of testes not palpable in the scrotum (impalpable) are found in the inguinal region; 20% are intra-abdominal and 10% are in an ectopic location.1 An impalpable testis may be absent in approximately 40% of boys as part of a testicular regression syndrome. This is usually secondary to intrauterine or perinatal torsion prior to fixation of the testis in the scrotum, and a testicular ‘nubbin’ or abnormal testicular remnant is the only tissue present.4 Hypertrophy of the contralateral testis is likely to occur. A useful comparison for the appropriate size of the testis is the size of the glans penis.3
Retractile testis
A retractile testis can be brought all the way into the scrotum without tension but retracts back to an inguinal position after a variable period. It is a normal variant. The cremaster muscle contracts to control the temperature of the testis, retracting it to the body when environmental temperature changes. When androgen levels are high at birth and at 3–6 months, the cremaster muscle is more relaxed. When androgen levels decrease after this, the muscle has a greater tendency to contract, causing retractile testes.3
Normal physiology of testicular descent
There are two stages of testicular descent, which occur at 8–15 and 25–35 weeks of gestation.2,3 The first stage is enlargement of the gubernaculum.3 If the first stage fails, the testis remains intra-abdominal.5 The second stage involves the gubernaculum extending into the scrotum, travelling approximately five centimetres, so the testis becomes intrascrotal.3 This stage is more commonly disrupted as it is more complex, leading to localisation of the testis between the deep inguinal ring and the scrotum.5
Emerging evidence suggests that UDT represents a disruption in the hormonally controlled testicular descent in fetal life and is probably secondary to a disturbance of intrauterine hormonal function.1,2 Important risk factors identified include maternal smoking, family history of UDT, low birth weight and prematurity.2 The prevalence in premature boys is up to 30.1%.6 Pregnant women can be reassured that the majority of other posited risk factors, such as maternal diet and caffeine consumption, intrauterine exposure to high levels of oestrogen, maternal use of medication and assisted reproduction, have not been supported with consistent evidence.2 Some studies have shown a correlation with environmental endocrine disruptors, but little is known about specific chemical exposures that may be causative.2
Diagnosis
Ask parents if the testis has ever been in the scrotum. Retractile testes often descend during a warm bath. An ectopic testis may appear as a lump in a different location.
Ideally, the physical examination should be done in a a warm environment and when the child is calm. A significant suprapubic fat pad can influence the examination and make a UDT difficult to detect. A well-developed hemiscrotum usually indicates an ascending or retractile testis. An undeveloped (hypoplastic) appearance indicates a possible UDT (Figure 1). It is important to recall that a UDT is often mobile as it remains within a patent processus vaginalis.3
Classify the testis as normal, high scrotal, supra-scrotal or impalpable (Figure 2).5 Ectopic testes can be in the perineum, femoral region, pre-penile region and even the opposite hemiscrotum.7
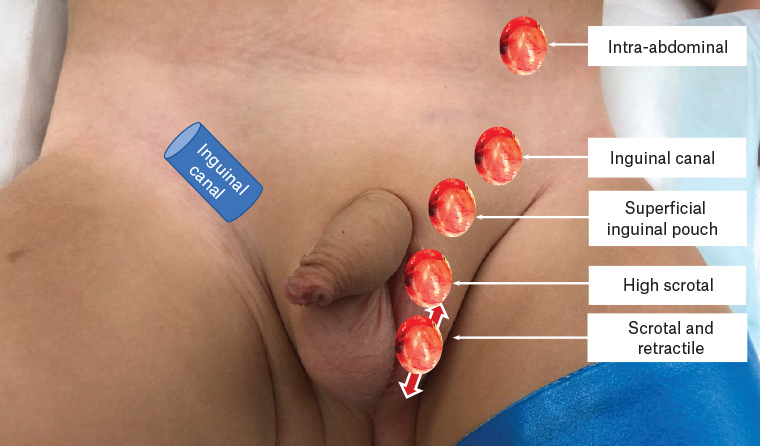
Figure 2. Possible anatomical positions of an undescended testicle
Investigations
Ultrasonography is not helpful prior to referral.5,7,8 It has a 45% sensitivity and 78% specificity in locating an impalpable testis.9 Paediatric surgeons will perform examination under anaesthesia and laparoscopy if unable to locate the testis on examination, facilitating diagnosis and management.
Management
Current guidelines recommend referral at 3–6 months for unilateral UDT, and orchidopexy between six and 12 months.1,7,8 If a testis appears to be ‘high’ (at the neck of the scrotum), the child needs yearly review as the testis may ascend.5 A referral pathway is suggested in Figure 3. Bilateral UDT or any additional genital ambiguity, such as hypospadias or bifid scrotum, warrant investigation for a disorder of sexual differentiation such as congenital adrenal hyperplasia, which may have lethal salt-wasting consequences.3,7,8
Examination under anaesthesia and diagnostic laparoscopy is the treatment for impalpable testes.1,8 If the testis is palpable within the inguinal canal or at the deep inguinal ring under anaesthetic, a single-stage operation can be successful in up to 90% of cases.10 If this is not possible, a two-stage procedure is performed (Fowler-Stephens procedure). The first stage involves intrabdominal high ligation of the testicular vessels with the blood supply now solely from the artery to the vas deferens. The second stage involves mobilisation of the testis into the scrotum. The overall success rate of this operation is 70–85%.3,6,11
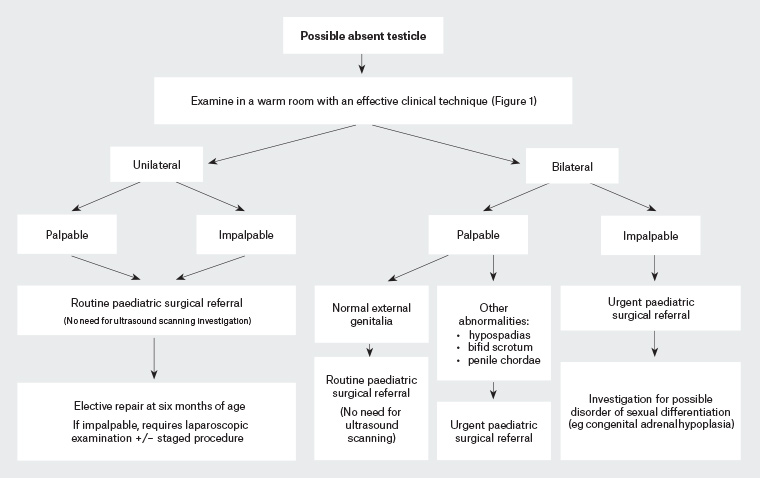
Figure 3. Investigation and treatment for undescended testicles in a newborn male infant
When an ascending testis is detected during childhood, the optimum time for orchidopexy is before puberty (usually around 7–8 years).3
If a testicular nubbin is found, there is some controversy around mandatory removal because of a potential risk of malignancy. There is insufficient evidence to suggest an inguinal or scrotal nubbin requires routine excision, as potentially malignant cells fail to persist as the child ages.4 Intra-abdominal nubbins may contain more elements and warrant excision.4
Hormonal treatment with gonadotrophin-releasing hormone or human chorionic gonadotropin has been investigated but has not proven efficacious in promoting testicular descent.3,8 Hormonal therapy can improve the fertility index in biopsies during orchidopexy, but there is no evidence of long-term fertility benefits.8
Complications and prognosis
Parents are often concerned about subfertility and the risk of malignancy. At birth, a UDT has germ cells but often in reduced numbers.12 A complete lack of germ cells has been reported as early as 15 months, hence the impetus to treat before the age of one year.12 The lack of germ cells is likely to be a result of exposure to a higher temperature (37°C) than that in the scrotum (34°C).3 The neonatal germ cell becomes a spermatogenic stem cell if it is in the scrotum at approximately 3–6 months and exposed to increased levels of androgens.3
The literature has shown there may be an association between UDT and infertility, but it does not appear to be as great as once feared or as parents are often told, particularly for unilateral UDT. In men with bilateral UDT, despite orchidopexy, the infertility rate may be up to 56%, six times that of the general population, and the paternity rate (the percentage of men attempting who successfully achieve conception) may be 62%.12–15 The prognosis for unilateral UDT appears to be better: 83% have a normal sperm count, and the paternity rate is up to 89%. Comparatively, the non-cryptorchid population has a paternity rate of 94%.12,14,15 Men with inguinal testes have a paternity rate of 90%, compared with a rate of 83.3% in men with intra-abdominal or canicular testes.16 In one study, the mean time to conception was not significantly different between unilateral UDT and controls, and the age at surgery did not alter paternity rates.17,18 Patients with unilateral UDT had half the sperm density of controls, but given the similar paternity rate, the contralateral testis probably produces most of the sperm.17 There is little evidence that orchidopexy improves fertility despite, in theory, improving the environment of the testis in the scrotum.19
The risk of testicular cancer has been approximated at 2% in UDT; although this is four to five times the risk in the general population, parents can be reassured this is still relatively low.12,20 A meta-analysis showed a relative risk of 4.8, and UDT is the only risk factor for testicular tumours that has level I evidence.20 The risk of malignancy in ascending testis is believed to be much lower.3 Boys with bilateral UDT appear to be at a higher risk than those with unilateral UDT, and the risk of cancer is usually highest in those with intra-abdominal testes, with the notable exception of complete androgen insensitivity.21 UDT is particularly associated with testicular seminoma, with a relative risk of 7.3.22 The tumours usually develop in young adults as germ cells rapidly multiply after puberty.21 One theory posits that the altered position of the UDT increases malignancy risk because of the changed environment of the testis. Some, but not all, evidence suggests men who undergo orchidopexy later in life have an increased risk of malignancy.23–25 Although there is no prospective evidence that orchidopexy reduces risk, bringing the testis into the scrotum does make detection of a cancer easier.
Some studies have shown an increased risk of malignancy in the contralateral testis in unilateral UDT.19,26 This supports a theory that the cancer risk may be from an overall syndrome of gonadal dysgenesis, of which UDT is a symptom, and relocating the testis does not reduce risk.20 To support this theory, other genital anomalies, such as hypospadias, increase the rate of testicular malignancy.22 There is thought to be an interruption to development of the germ cells in utero. These cells often have the appearance of carcinoma in situ cells and can undergo a malignant transformation.21 However, there is still much to be understood about UDT in the context of malignancy, and there is a lack of prospective data.
Key points
- UDT is a common disorder that affects 1–2% of boys and should be referred at three months of age.
- Investigations are not required prior to referral.
- Surgery remains the treatment of choice.
- Decreased fertility is largely a concern for bilateral UDT. Malignancy occurs at a higher rate but remains relatively low risk.