A difference in pupil size between the eyes is known as anisocoria. The aetiology may be physiological, pathological or pharmacological. The general practitioner (GP) may discover anisocoria during examination for a seemingly unrelated problem. Indeed, new onset anisocoria may be an early sign of a life-threatening emergency.1 This article aims to guide management in both of these situations.
Physiological anisocoria is common: approximately 20% of normal people have different-sized pupils.2–4 Non-physiological anisocoria indicates disease of the sympathetic or parasympathetic pathways supplying the pupil, or a problem with the iris itself.2,5
A thorough history should include asking about the use of new medications or inadvertent ocular contact with foreign substances by rubbing the eyes. Associated visual and/or neurological symptoms should be sought, including visual blurring, visual loss, disturbance of visual fields, or diplopia.5 It is important to ask about previous or current malignancies and neck trauma. Examination should include assessment of visual acuity, visual fields to confrontation, pupil testing, extraocular motility and whether or not ptosis is present.2
Pupillary pathways
The pupillary light response involves both afferent (optic nerve) and efferent (oculomotor nerve and sympathetic) pathways.2 Information from the optic nerve passes to the ipsilateral pretectal nucleus and then on to the Edinger-Westphal nuclei on both sides.5 It is this bilateral innervation of the Edinger-Westphal nucleus that results in both direct and consensual responses to light shone in one eye.
Parasympathetic pupilloconstrictor fibres travel in the oculomotor nerve, synapsing in the ciliary ganglion before reaching the sphincter pupillae muscle in the iris.2 Sympathetic pupillary fibres originate in the hypothalamus, travel down the brainstem and cervical spinal cord to exit at the first thoracic level.5 From here, second-order sympathetic neurons travel back up the sympathetic chain to synapse in the superior cervical (stellate) ganglion.2,5 Third-order sympathetic neurons then travel to the orbit with the internal carotid artery and its branches, ultimately innervating the dilator pupillae muscle in the iris.2,5
Direct and consensual responses should be identical, whichever eye is illuminated. Testing this forms the basis of the swinging-torch test: if the response to shining a light in one eye differs from shining it in the other, this is referred to as a relative afferent pupillary defect (RAPD).6
Examination check list
The examiner should observe the shape, size and location of the patient’s pupil. Any asymmetry of the colour of the two irides (heterochromia) should be noted. A baseline measurement of pupil size should be made under ambient lighting conditions with both pupils equally illuminated. If the patient’s irides are dark and the pupils are difficult to visualise, the clinician can use an ophthalmoscope to assess differences in the red reflex.
Pupil size should be assessed in both the dark (sympathetic-dominated dilation) and the light (parasympathetic-dominated constriction). If anisocoria remains the same in both lighting conditions, and the difference between pupil sizes is no more than 2 mm, the aetiology is likely to be physiological.6 The presence of ptosis and/or extraocular muscle involvement suggests a pathological process regardless of the magnitude of anisocoria.
The response to testing each eye separately with a bright light source is then assessed. The clinician should observe the direct pupillary response in the illuminated eye and the consensual response in the fellow eye. The swinging-torch test (for an RAPD) is then performed, ideally in a darkened room with a very bright light.6 One eye is illuminated for two seconds and the response of both pupils is assessed. The light is then redirected across the nose as quickly as possible to the other eye and, again, the response of both pupils is assessed before returning to the first eye.
If an RAPD is present, the response of both pupils will be less when the light is shone in one eye in comparison to the other.6 The pupils will constrict as the light is moved to the better eye, and dilate when the light is moved back to the worse eye.6 Note that the swinging-torch test identifies a defect in the afferent visual pathway, such as the optic nerve; pupillary reactions in the presence of parasympathetic or sympathetic lesions (efferent lesions) remain present, independent of which eye is illuminated.
Finally, the response of both pupils to accommodation is assessed by getting the patient to fixate on a target held in front of both eyes and gradually moved towards the patient, up to the limits of convergence.
Diagnostic criteria
A misshapen and/or asymmetrical pupil is usually due to disease of the iris (Figure 1). Common causes for iris-related anisocoria include previous corneal or cataract surgery, posterior synechiae (adhesions to the lens) from previous uveitis, or ocular injury (traumatic mydriasis).2 Anisocoria associated with a unilateral red eye, pain and blurred vision may indicate uveitis and warrant ophthalmology referral. Rare pupillary abnormalities include a white pupil (leukocoria). The presence of leukocoria in children is a red flag for retinoblastoma and requires urgent referral. Red flags of which to be aware when assessing a patient with anisocoria are listed in Table 1. Figure 1 illustrates a flow chart to help clinicians determine the likely cause of anisocoria and arrange expeditious referral where appropriate.
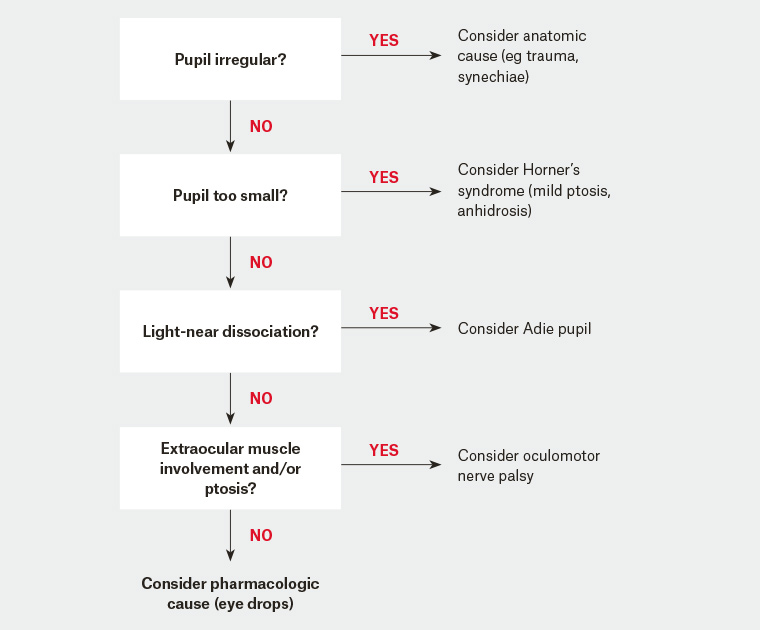
Figure 1. Flow chart for an assessment of anisocoria
|
Table 1. Red flags for unequal pupils
|
|
Anisocoria in the light (larger pupil abnormal)
Larger pupil does not constrict briskly to bright light
|
|
Associated clinical findings
|
Likely diagnosis
|
|
Ipsilateral ptosis
|
Oculomotor nerve palsy
|
|
New onset diplopia with ipsilateral ocular motility disturbance
|
Oculomotor nerve palsy (life-threatening emergency)
|
|
Signs or history of previous ocular trauma
|
Traumatic mydriasis from damage to the sphincter pupillae in the iris
|
|
Anisocoria in the dark (smaller pupil abnormal)
Both pupils may still constrict briskly to bright light
|
|
Associated clinical findings
|
Likely diagnosis
|
|
Ipsilateral (partial) ptosis
|
Horner’s syndrome
|
|
New onset diplopia with ipsilateral ocular motility disturbance
|
Horner’s syndrome with additional oculomotor nerve lesion
(eg lesion in the cavernous sinus)
|
|
Ipsilateral red eye with pain and ciliary hyperaemia
|
Acute anterior uveitis
|
|
Ipsilateral face or neck pain
|
Horner’s syndrome secondary to internal carotid artery dissection
(life-threatening emergency)
|
|
Ocular contact with foreign substances by rubbing the eyes
|
Pharmacologically induced anisocoria
|
Anisocoria due to failure of constriction of one pupil is most obvious in bright light. Therefore, if anisocoria is greater in bright illumination, the underlying cause of the failure to constrict could be a tonic (Adie’s) pupil, an oculomotor nerve palsy or, possibly, drug-induced mydriasis.2,7 An Adie’s pupil is the result of disease of the ciliary ganglion and is typically characterised by light-near dissociation in which the pupil fails to constrict to light but does constrict slowly to a near target as the result of accommodation.2 The ‘near’ pupillary constriction can be difficult to elicit in elderly patients in whom accommodation is reduced.
A dilated pupil arising from an oculomotor nerve palsy is almost always associated with some abnormality of eyelid and/or extraocular muscles. Of those patients presenting with an acquired oculomotor palsy, only 6% were found to have compression from an aneurysm and, of these compressive oculomotor nerve palsies, only 64% had pupil involvement.8 However, all patients with a new-onset oculomotor nerve palsy should be presumed to have a posterior communicating artery aneurysm until proven otherwise, meaning they require urgent neuroimaging.2
Conversely, failure of dilation is most obvious in the dark. Anisocoria that is greater in the dark implies failure of dilation and the cause is likely to be a sympathetic palsy (Horner’s syndrome) or, possibly, the use of parasympathomimetic medication (eg pilocarpine). Horner’s syndrome may result from a lesion anywhere along the sympathetic pupillary pathway.9 For example, it may be caused by a brainstem stroke, cervical spondylosis, an apical lung tumour, a dissection of the internal carotid artery (ICA), or surgery to the chest or neck.2,9
The timing of onset and associated symptoms allows the clinician to determine the urgency for further investigations. Interestingly, Almog et al found the pathology underlying a Horner’s syndrome was apparent in >80% of patients after one consultation on the basis of clinical history and examination alone.10 Importantly, when a new-onset Horner’s syndrome is seen in the context of brain stem or cerebellar signs, this should be a red flag for a brain stem or cerebellar stroke.9 When the signs of Horner’s are associated with a hoarse voice, this is a red flag for a compressive lesion in the chest or neck.2 Alternatively, the development of a painful Horner’s should suggest an ICA dissection (over half of patients with an ICA dissection present with a painful Horner’s syndrome).11,12 Consequently, any patient presenting with acute onset Horner’s and ipsilateral ocular, face or neck pain should be regarded as an ICA dissection until proven otherwise.11 If no localising signs, pain, trauma or malignancy are present, and the Horner’s syndrome is of non-acute onset, it is reasonable to investigate on a routine basis.13
The location of the lesion within the sympathetic pathway is classified as central, preganglionic or postganglionic, corresponding to the first-order, second-order and third-order neurons above. A complete Horner’s syndrome comprises miosis on the affected side, along with ipsilateral partial ptosis due to paralysis of the sympathetically innervated superior tarsal muscle (Müller’s muscle).9 There may also be ipsilateral anhidrosis of the face.9 Note, ptosis has reportedly been absent in up to 12% of patients with Horner’s syndrome.14
Relative afferent pupillary defect
An RAPD implies a lesion of the afferent pathway.2 The cause is usually disease of the optic nerve, but it can be seen in severe, asymmetric retinal disease (Box 1). Patients with an RAPD do not have anisocoria unless there is an associated efferent lesion of one eye. Great care should be taken when assessing for the presence of an RAPD, as this may be the only sign of a lesion within the afferent pupillary pathway.15
|
Box 1. Aetiology of a relative afferent pupillary defect
|
|
Relative afferent pupillary defect present
|
|
Asymmetrical optic nerve disease:
Extensive asymmetrical retinal disease:
|
Conclusion
The aetiology underlying anisocoria may be physiological, pathological or pharmacological. The clinician must determine whether a patient with anisocoria can be reassured or, instead, requires referral for further investigation. If any uncertainty exists, onward referral is appropriate granted the potential for serious underlying systemic pathology.