Colorectal cancer (CRC) is the second most common cancer, after breast cancer, in Australia.1 After lung cancer, it is the second most common cause of cancer death.1 Statistics from the Australian Institute of Health and Welfare (AIHW) estimate that in 2018, 17,004 new cases of CRC will be diagnosed.1
It is well established that CRC has a precancerous stage in the form of a polyp, which acquires mutations through one of three molecular pathways to become an invasive lesion.2 These molecular pathways include the conventional adenoma–carcinoma sequence (APC gene mutation),2 the serrated pathway (KRAS and BRAF mutations) and the familial pathways (eg Lynch syndrome and familial adenomatous polyposis).3 The objective of screening in CRC is to identify individuals who have a higher risk of harbouring a polyp or early cancer (via detection of blood in stool) and thus to remove precancerous lesions (ie polyps) or early cancer in order to stage-shift cancer detection to less advanced stages, and thus reduce mortality. Given this pathophysiological process, the screening of CRC remains one of the most effective screening methods available for detecting cancer.4
Tools used to screen for colorectal cancer
The current guidelines suggest using a faecal occult blood test (FOBT), which aims to identify microscopic blood in the stool, as the first-line screening test to detect CRC. There are two forms of the test: the guiac-based form (gFOBT) and the immunochemical form (iFOBT). The gFOBT identifies haemoglobin in the stool through a peroxidase reaction of the haem component of haemoglobin.5 Importantly, this reaction is not limited just to human haem, and can be positive following the ingestion of red meats.5 The iFOBT uses an antibody reaction to specifically identify the human haemoglobin.5 Since the gFOBT is not as specific as the iFOBT, diet and medication modifications, such as avoiding red meat, vitamin C and anti-inflammatories, are required three to five days prior to testing.5 As such, iFOBT is the preferred test. In patients who have macroscopic blood loss, a referral for colonoscopy should be undertaken without using FOBT. Other screening tests, such as multi-targeted DNA stool testing, flexible sigmoidoscopy and colonoscopy, are also available but are not part of the current screening program because of cost, resource limitations and risks associated with invasive procedures.6
Evidence for the screening of colorectal cancer
One of the earliest sources of evidence highlighting the benefits of CRC screening was published in the New England Journal of Medicine in 1993, when a study cohort of 46,551 participants aged 50–80 years was assigned to annual gFOBT, biennial gFOBT or a control group.7 Through the use of gFOBT, it was found that the 13-year cumulative mortality from colorectal cancer was reduced by 33%.7 A recent systematic review and meta-analysis by Fitzpatrick-Lewis et al8 in 2016 comparing screening through gFOBT, iFOBT, flexible sigmoidoscopy and colonoscopy showed no difference in all-cause mortality between the screening methods. The pooled results from the four randomised studies using gFOBT compared with controls showed an 18% reduction in disease-specific mortality with a median follow-up of 18.25 years.8 The results from the only randomised controlled trial (RCT) available using iFOBT showed no difference in mortality, although the length of follow-up was only six years, much shorter than the 10-year interval expected for screening results to show benefit.8
Studies have also characterised the rates of detection of advanced colorectal adenoma and early and advanced CRC using the iFOBT in average-risk populations. A Japanese study in 2010 examined the detection rates of iFOBT in 700 patients who were undergoing concurrent screening with a colonoscopy.9 Patients were deemed to be at average risk of developing CRC. The sensitivity of the iFOBT in being able to detect pre‑cancerous adenomas was 33.9%, and specificity was 90.6%.9 In detecting early stage CRC, the sensitivity was 84.6% and specificity was 89.8%9. In advanced CRC, the sensitivity was 43.7% and specificity 91.9%9.
The National Bowel Cancer Screening Program
The National Bowel Cancer Screening Program (NBCSP) was introduced in 2006. By 2020, all Australians aged 50–74 years will be offered screening every two years. While the iFOBT is mailed out to patients as part of the program, general practitioners (GPs) can request these tests for patients who have missed out. The AIHW has recently released a report on the national screening (Box 1).10
| Box 1. Summary of the Australian Institute of Health and Welfare’s national report on bowel cancer screening for 201710 |
- A total of 2.6 million people were invited to participate between 2014 and 2015
- There was a 39% participation rate
- Re-participation rate for those who had previously taken part and were receiving a subsequent screening invitation was 76%
- In 2015, 41,000 of the population screened had a positive faecal occult blood test (FOBT) – 8% positivity rate
- Of those with a positive test, 70% had follow-up investigation with the median time interval of 53 days
- Of the patients with positive FOBT, 58% will have normal colonoscopy, 39% will be diagnosed with a polyp and only 3% will be diagnosed with cancer or suspected cancer
- Risk factors for positive FOBT screening included: male gender, first screening, older ages, remote communities, low socioeconomic status and Aboriginal or Torres Strait Islander background
- Similarly, individuals are less likely to participate if they are young; have an Aboriginal or Torres Strait Islander ancestry, non–English speaking background, male gender or low socioeconomic status; or live in remote regions
|
Data linkage studies have shown a reduction in both mortality and morbidity associated with CRC since the introduction of screening.11 This trend of improved outcomes is only partly attributed to screening, as the decline predated the introduction of national screening and the magnitude of change is much more than would be expected with screening alone. Welch et al12 suggested three possible explanations:
- improved surgical techniques, standardisation of preoperative and postoperative care and, importantly, the addition of adjuvant chemotherapy has improved survival
- population education and bowel cancer awareness
- healthier lifestyles and dietary changes.
Thus, it is important that GPs stipulate to patients that the best way to reduce mortality and, in particular, incidence (as there is no evidence that screening reduces incidence) is to adopt healthier lifestyles. This would include advising patients to reduce or cease smoking, limit alcohol intake, exercise regularly and consume a balanced diet.13
Recommendations
Updates in CRC screening guidelines
The current recommended strategy for population screening in Australia is an iFOBT every two years in asymptomatic individuals, starting from age 50 years to age 74 years.4 Extending the screening beyond the age of 74 years is not recommended as it is not cost-effective.4 In people aged 45–49 years who request screening after being fully informed of the advantages and harms of testing, an iFOBT every two years should be offered during the lead-up to their first routine invitations by the NBCSP at age 50 years.4 GPs have a vital role in identifying and advising individuals to opt out of the screening program if they have major comorbidities and limited life expectancy or if they have had a recent major illness or surgery. Several factors have been identified as barriers to FOBT uptake, including inconvenience, perceptions of handling faeces, fear of cancer diagnosis and cultural practices.14 GPs are crucial in addressing these modifiable factors and improving uptake of the screening program, which currently sits at 39%.10 As shown by Lew et al,15 at this current participation rate, the NBCSP is predicted to prevent 92,200 cancer cases and 59,000 cancer-related deaths over the period between 2015 and 2040.15 An additional 24,300 cancer cases and 37,300 deaths would be prevented if this participation rate was increased to 60%.15
Individuals who are symptomatic should not participate in the screening program but be referred directly for appropriate investigation. This also pertains to high-risk individuals such as those with recent history of colorectal cancer, chronic inflammatory bowel disease or certain high-risk genetic disorders.4 For patients who have had a recent high-quality colonoscopy (within two years), the current recommendation is that screening should be deferred for another two years.16 One positive iFOBT is adequate to refer for colonoscopy; repeating a positive FOBT is not recommended.16 Given patients’ fear of cancer diagnosis in the setting of a positive FOBT, it is often important to reassure this cohort that over 50% will have a normal colonoscopy.10
Risk and screening based on family history
Changes in risk stratification on the basis of family history are outlined in Table 1. It is important for health practitioners to investigate family history thoroughly to properly ascertain a patient’s risk. A family history of CRC is a significant risk factor for developing the disease.
| Table 1. Risk stratification based on family history24 |
Category 1
(relative risk x 1–2) |
Category 2
(relative risk x 3–6) |
Category 3
(relative risk x 7–10) |
Asymptomatic people who have:
- no personal history of bowel cancer, colorectal adenomas, inflammatory bowel disease or family history of colorectal cancer (CRC)
OR
- one first-degree relative with CRC diagnosed at 55 years or older
OR
- one first-degree and one second-degree relative with CRC diagnosed at 55 years or older.
|
Asymptomatic people who have:
- one first-degree relative with CRC diagnosed before age 55 years
OR
- two first-degree relatives with CRC diagnosed at any age
OR
- one first-degree relative and at least two second-degree relatives diagnosed with CRC at any age.
|
Asymptomatic people who have:
- at least three first-degree or second-degree relatives with CRC, with at least one diagnosed before age 55 years
OR
- at least three first-degree relatives with CRC diagnosed at any age.
|
The screening strategies for those at an increased risk of developing colorectal cancer have also changed.
Important changes include the age of commencing screening in patients with a family history and the recommendations for the use of aspirin. For all people aged 50–70 years, regardless of their risk of developing CRC, GPs should now actively consider commencing patients on low-dose aspirin (100–300 mg).17 There is level 1 evidence to suggest that regular consumption of aspirin can reduce incidence and mortality in CRC.18 Pooled analysis from RCTs has shown that daily aspirin use can reduce the occurrence of CRC by 24% and reduce CRC-associated mortality by 35% after 10 years of taking the drug.19 The mechanism by which aspirin to prevent the development of CRC is unknown. Aside from antiplatelet effects, it has been hypothesised that aspirin may be involved in altering tumorigenesis through the modulation of cyclooxygenase-2, which has an important role in cellular proliferation and angiogenesis.20,21 Additionally, aspirin is known to provide benefits in cardiovascular and neurovascular disease, as well as being cheap and readily accessible.
The safety profile of aspirin, given its widespread use, is reasonably well understood. The most serious adverse effects associated with regular aspirin use are gastrointestinal and intracranial bleeding. A meta-analysis of 35 RCTs found that a daily aspirin dose between 75 and 325 mg was associated with a hazard ratio for a major gastrointestinal bleed of 1.55 (95% confidence interval [CI]: 1.27, 1.90) in comparison to the placebo group.22 The risk of intracranial bleeding associated with low-dose aspirin has been shown to be lower than the risk of gastrointestinal bleeding but deemed significant because of its complications.23 Some, but importantly not all, studies have noted that higher doses have been associated with higher incidences and complications of gastrointestinal and intracranial bleeding. Further studies are needed to clarify the dose and duration of use for aspirin to be most effective. The current recommendation is that patients taking aspirin for prevention of CRC should do so for at least 2.5 years.17 There has been no evidence to suggest that a higher dose of aspirin, such as 600 mg, is more effective in reducing CRC mortality.17
Of relevance for general practice is that the recommendations for screening do not apply to patients who are symptomatic or who have a personal history of CRC. In such cases, referral to a specialist is advised.
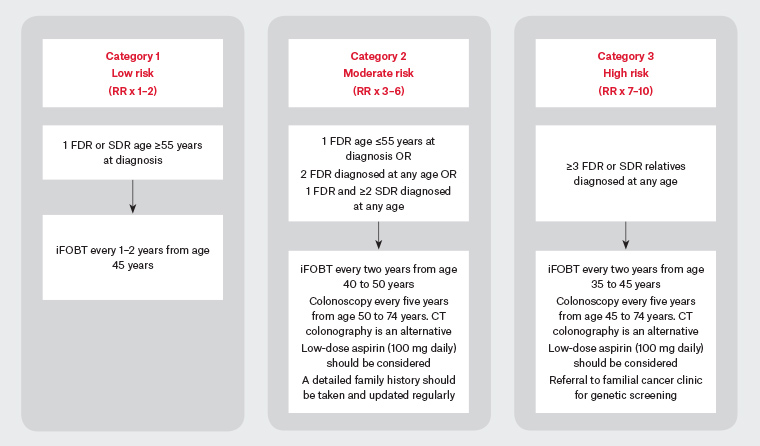
Figure 1: Screening flowchart based on family history17,24,25
CT, computerised tomography; FDR, first-degree relative; iFOBT, immunochemical faecal occult blood test; RR, relative risk; SDR, second-degree relative
| Table 2. Current screening guidelines based on family history17,24 |
| Category 1 (relative risk x 1–2) |
Category 2 (relative risk x 3–6) |
Category 3 (relative risk x 7–10) |
- Immunochemical faecal occult blood test (iFOBT) should be considered every two years from age 45 years, given the risk of colorectal cancer at this age is approximately equivalent to the population risk at age 50 years
- For patients aged 50–70 years, low-dose aspirin (100 mg) daily should be considered
|
- iFOBT should be performed every two years from age 40 to 50 years
- Colonoscopy should be performed every five years from age 50 to 74 years. Computed tomography (CT) colonography can be offered if colonoscopy is contraindicated
- Low-dose (100 mg) aspirin daily should be considered
- As a result of the possibility of Lynch syndrome, a complete family history should be taken and updated regularly, and the accuracy of the cancer diagnoses and polyp pathology should be checked carefully
- Genetic testing is not appropriate at present for people with category 2 risk
|
- iFOBT should be performed every two years from age 35 to 45 years
- Five-yearly colonoscopy from age 45 to 74 years. CT colonography can be offered if colonoscopy is contraindicated
- Low-dose (100 mg) aspirin daily should be considered
- Referral to a genetic centre for hereditary cancer syndromes should be considered
|
Conclusion
CRC remains a huge health burden in Australia, and screening is vital in reducing mortality.4 The major limitation in the screening program is the low participation rate.10 GPs have a crucial role in the success of screening by facilitating participation in the screening program. Screening of patients with symptoms is inappropriate. Instead, these patients should be referred for investigation of their symptoms. Determining the patient’s risk, providing education, encouraging screening and instituting prompt referrals are essential in reducing CRC morbidity and mortality. The updated guidelines provide clear guidance regarding the current screening recommendations and suggest biennial screening between the ages of 50 and 74 years. The use of low-dose aspirin should now be discussed with all patients aged 50–70 years as a form of chemoprophylaxis. Individuals with a family history of CRC will need to start screening at an earlier age on the basis of category of risk. Further details regarding screening and management of CRC can be found on the Cancer Council Australia website (https://wiki.cancer.org.au/australia/Guidelines:Colorectal_cancer).