Nocturia, defined as waking up from sleep to pass urine, is rarely benign. Individuals who void at least twice per night have over four times the risk of developing cardiovascular disease and double the risk of early death, even when controlled for known risk factors for mortality.1 Health issues, such as diabetes, hypertension, cardiovascular disorders, mental health problems and obesity, are more common in people with nocturia, generating up to a threefold increase in the use of healthcare services.2–5
Figure 1 summarises the possible underlying causes of nocturia, many of which co-exist, interact and lie outside the urinary tract system.6–8 There is an age-related increase in nocturnal urine production in older people that can interact with reduced bladder sensation and poor bladder emptying. Patients with nocturia will usually have a mismatch between nocturnal urine production and bladder storage.9 Alternatively, they may have insomnia or sleep disturbance.
The aim of this paper is to provide primary care practitioners with eight questions to ask to guide diagnosis, comprehensive evaluation and individualised treatment of nocturia.
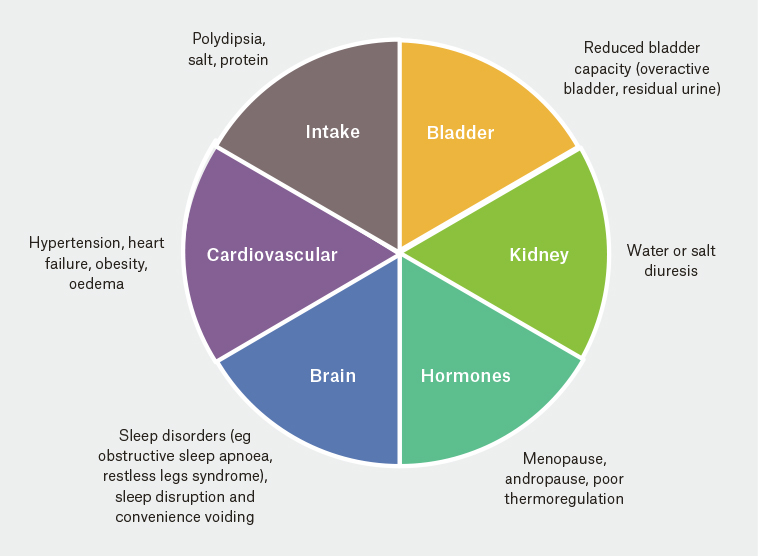
Figure 1. Underlying causes of nocturia
History taking
The patient-completed nocturia screening tool, Targeting the individual’s Aetiology of Nocturia to Guide Outcomes (TANGO;10 Appendix 1), allows the general practitioner (GP) to consider possible causes of increased overnight urine production, sleep dysfunction and sleep disturbance; identify the presence of an overactive bladder (OAB) and possible voiding dysfunction; and clarify the impact of nocturia. The GP can then gain additional relevant information by asking the following leading questions.
Question 1: How many times do you wake up at night to pass urine?
The onset and duration of nocturia, number of awakenings at night and relative bother should be clarified. In particular, waking from sleep with an urge to void should be differentiated from voids made ‘just in case’, which are common in those who wake because of sleep disturbance.
Question 2: How much does nocturia bother you?
Persistent nocturia signals impair sleep quality for most patients, usually reducing the time per cycle spent in slow-wave sleep. Many older patients do not automatically associate nocturia with their poor sleep, although the symptoms drive each other. Recently, the first undisturbed sleep time (FUST) before awakening to void has been described as significant to patients. Interruption early in the night causes fatigue the next day and over time affects functioning and quality of life.11,12 A FUST of three hours or less is associated with high bother for most patients with nocturia.
Question 3: What medications are you taking?
A review of the patient’s medications is important, especially when onset of nocturia coincides with recently commenced pharmacotherapy. Medications may contribute to nocturia by inducing diuresis or disturbing sleep. A clinical overview of diuresis and medication is summarised in Table 1. Concurrent medication is also important when introducing antidiuretic therapy.
|
Table 1. Impact of medications on nocturia mechanisms
|
|
Mechanism
|
Drugs
|
|
Increase free water clearance (diuresis)
|
Diuretics, progesterone, melatonin
|
|
Increase osmotic clearance (diuresis)
|
All diuretics, ACE inhibitors, lithium, progesterone, SGLT-2 inhibitors
|
|
Decrease free water clearance (antidiuresis)
|
dDAVP, oestrogens, testosterone, antipsychotics, chemotherapeutics, antidepressants, antiepileptics, older glucose-lowering medications, opiates
|
|
Decrease osmotic clearance (antidiuresis)
|
Calcium channel blockers, beta adrenoceptor antagonists, NSAIDs, lithium, oestrogen, testosterone, melatonin, corticosteroids, thiazolidinediones
|
|
Induce postural hypotension
|
Hypotensive drugs, anti-Parkinson's medication, older psychotropic agents, thiazides
|
|
Induce oedema
|
Calcium channel blockers, steroids, NSAIDs
|
|
Sleep disruption
|
Antiepileptics, decongestants, SSRIs, SNRIs, caffeine
|
|
Bladder stimulation
|
Caffeine, alcohol, anticholinesterase inhibitors, cyclophosphamide, ketamine
|
|
ACE, angiotensin converting enzyme; dDAVP, desmopressin acetate; NSAIDs, nonsteroidal anti-inflammatory drugs; SGLT-2, sodium–glucose co-transporter 2; SNRIs, serotonin and noradrenaline reuptake inhibitors; SSRIs, selective serotonin reuptake inhibitors
|
Question 4: How much is your bladder actually storing day and night?
A bladder diary (or frequency volume chart [FVC]) documenting intake and voiding over 48–72 hours is essential to understand whether the patient has normal or restricted bladder storage. It is often a tedious task for patients and may be poorly completed; however, findings do form the backbone of diagnosis. Collection containers with marked volumes, which fit under a toilet seat and decrease patient burden, are available. The FVC may be web-based or a paper diary and should, ideally, be in the patient’s preferred language.
OAB can be associated with reduced day and night voided volumes, urinary urgency and frequent micturition. In OAB, voiding frequency occurs up to eight times per day and voided volumes are usually <300 mL during the day. Frequent urination and low voided volumes may also be due to incomplete emptying, with increased post-void residual (PVR) volumes. Elevation of PVR urine volumes is particularly likely with longstanding diabetes mellitus, previous urinary retention, urinary tract infections, chronic constipation, known detrusor underactivity or bladder outlet obstruction. Ultrasonography is recommended to exclude an elevated PVR.
Table 2 shows a sample FVC and diagnostic measures derived from the data. The data indicate nocturnal polyuria and suggest the patient is likely to have OAB. There is no evidence of global polyuria, polydipsia or overnight drinking.
|
Table 2. Example of one day from a frequency volume chart
|
|
Time
|
Type of drink
|
Amount of drink (mL)
|
Time
|
Amount of urine passed (mL)
|
|
8.30 am
|
Tea
|
200
|
9.00 am
|
100
|
|
9.00 am
|
Water
|
100
|
10.00 am
|
80
|
|
|
|
|
10.45 am
|
90
|
|
11.30 am
|
Tea
|
200
|
11.30 am
|
70
|
|
12.30 pm
|
Soup
|
200
|
12.15 pm
|
50
|
|
2.00 pm
|
Tea
|
200
|
2.00 pm
|
80
|
|
3.00 pm
|
Water
|
250
|
3.30 pm
|
100
|
|
4.30 pm
|
Tea
|
200
|
5.00 pm
|
80
|
|
6.30 pm
|
Water
|
250
|
6.45 pm
|
70
|
|
8.00 pm
|
Tea
|
200
|
8.30 pm
|
50
|
|
10.00 pm
|
Water
|
100
|
10.15 pm BED
|
50
|
|
|
|
|
1.00 am
|
300
|
|
|
|
|
3.00 am
|
350
|
|
|
|
|
5.30 am
|
400
|
|
|
|
|
8.00 am WAKE
|
100
|
|
Using the frequency volume chart data:
- 24-hour urine production = 1970 mL (first void included in previous night)
- Hourly urine production = 93 mL
- Nocturnal urine production = 1150 mL
- Nocturnal Polyuria Index = 58% (ie 1150/1970): cut-off normal = 33%
- Diuresis rate during day = 65.7 mL/hour
- Diuresis rate at night = 150 mL/hour
- Mean voided volume ~ 100 mL
- First undisturbed sleep time = 2.5 hours
|
Question 5: How much urine do you make over night?
A bladder diary identifies whether the patient has either global or nocturnal polyuria. The primary measure is the amount of urine produced between falling asleep and emptying the bladder when waking the next day. Urine output occurring overnight that exceeds 33% of 24-hour urine production indicates nocturnal polyuria; this is a likely finding in up to 80% of patients with nocturia.13 Global polyuria is diagnosed when 24-hour urine production exceeds 40 mL/kg body weight (commonly seen with diabetes mellitus, diabetes insipidus and polydipsia). Nocturia occurs when nocturnal urine production exceeds the bladder capacity.
Question 6: Do you have hypertension or cardiac failure, with or without leg oedema?
Blood pressure rises when supine, with increasing renal perfusion, potentially resulting in nocturnal polyuria.14 Antihypertensive medication taken earlier in the day may not facilitate dipping of blood pressure during the night. Swapping treatment for high blood pressure to the evening may reduce the volume of urine produced overnight.
Patients with accumulation of dependent oedema will reabsorb this third-space fluid when supine. For most people, this occurs at night and increases renal perfusion, resulting in nocturnal polyuria. A morning dose of furosemide may not prevent evening peripheral oedema; however, the same medication taken six hours before bed may induce evening voiding and thereby reduce fluid accumulation.
Question 7: Are you a good sleeper?
Obstructive sleep apnoea induces negative intra-thoracic pressure, resulting in raised blood pressure and nocturnal polyuria. Common symptoms are snoring loudly, episodes of suffocation during sleep and excessive sleepiness during the day. Other relevant causes of sleep disturbance include insomnia, restless legs, skeletal pain, anxiety, depression and bedroom environmental factors. Sleep disruption, regardless of the cause, is linked to nocturnal polyuria.15
Question 8: Have we checked your hormone levels recently?
The net effect of all sex hormones is antidiuresis at night.16 In women, nocturia occurs more often in the presence of hot flushes or after a hysterectomy.17 Loss of oestrogen is also a risk factor for sleep disturbance and impaired wellbeing. As yet there is no clinical evidence that hormonal substitution in postmenopausal women changes nocturia, although vaginal oestrogens can improve OAB and urinary tract infections.
Depletion of testosterone is associated with loss of muscle mass and bone strength, reduced bone mineral density, sexual dysfunction, deterioration of insulin sensitivity, elevated visceral fat and metabolic syndrome, all of which are on the causal pathway of nocturia.18 Loss of testosterone also alters circadian rhythms, thermoregulation during the night and sleep quality. Testosterone production may be impaired by interrupted deep sleep or sleep apnoea.19 The mechanism is likely to be related to reduced vasopressin levels, preventing reduction in night diuresis. It is not known whether testosterone supplementation reduces nocturia.20
Examination and testing
A general physical examination will identify any underlying disorders that may be exaggerating the mismatch between overnight urine production and bladder storage, or blunting the arousal mechanisms. Clinically relevant findings include:
Potential causes of nocturia, such as diabetes, heart, liver or kidney disease or sex hormone depletion, should be identified from routine blood tests.
Targeted treatment
Using the TANGO screening tool and the critical questions, it will be possible to identify:
-
global or nocturnal polyuria
-
size of mismatch between urine produced overnight and bladder storage
-
medical reasons underlying increased urine production at night
-
medical reasons inducing reduced voided volumes
-
causes of sleep disturbance
-
presence and interaction of multiple comorbidities
-
drugs that may be inducing urine production.
Current treatment options are summarised in Table 3. The lack of subclassification of nocturia into patients with and without nocturnal polyuria in clinical trials limits evidence of treatment efficacy. Heterogeneity and poor reporting of clinical endpoints relevant to nocturia limit current evidence to support efficacy of lifestyle interventions, alpha-adrenoceptor antagonists, antimuscarinic therapy, anti-inflammatory drugs and melatonin.21 For this reason, current treatment recommendations for nocturia are based on evidence from controlled trials or cohort studies.
|
Table 3. Intervention strategies for nocturia
|
|
Optimise general health
|
|
Bladder rehabilitation
-
Drug therapy for OAB
-
Bladder retraining for OAB
-
Reduction of bladder irritants
-
Anxiety management strategies
|
|
Nocturnal urine volume reduction
-
Prevent/decrease lower leg oedema
-
Fluid intake adaptation
-
Change in timing of diuretic (six hours before bed)
-
Antihypertension medication at night
-
Dietary restriction for sodium ± excessive protein at night
-
Antidiuresis pharmacotherapy at night
|
|
Sleep prolongation
|
|
DM, diabetes mellitus; OAB, overactive bladder
|
Treatments will differ between patients and will frequently be a combination of interventions. Targeting therapies for individual aetiological factors is necessary to address each patient’s modifiable causes of nocturia.22 Where the TANGO has highlighted medical issues related to increased diuresis, attention will focus on optimising management of these comorbidities. Treatment of confirmed sleep-disordered breathing is particularly promising for the resolution of nocturia.23 Management of daytime lower urinary tract symptoms, with or without antimuscarinic or beta-adrenoceptor agonist medication, may increase nocturnal bladder capacity. Antidiuresis therapy is effective for treatment of nocturia where there is evidence of nocturnal polyuria.21 Treatment efficacy can be evaluated by a positive change in nocturia frequency and patient-reported bother.
For individuals with persisting nocturnal polyuria, the only options are desmopressin (Level 1a evidence) and furosemide (Level 1b evidence).24 However, both medications potentially cause hyponatraemia, which has resulted in a warning or contraindication in people over the age of 65 years. Lowering the dose of desmopressin has proven to be safe in older people when there is a normal baseline serum sodium, an estimated glomerular filtration rate of >60 mL/min/1.73m2 and the absence of congestive cardiac failure or marked leg oedema. The scientific rationale for a gender-specific dose relates to a higher presence of vasopressin V2 receptors in females, who respond to a lower dose than males.
If diuretic therapy is also needed during the day, a short-acting (loop) diuretic is preferable to a longer acting (thiazide) formulation. Antidiuresis treatment with furosemide should be given at least six hours before bed to clear third-space fluid and minimise effect on nocturnal urine production. Day diuretic therapy should precede vasopressin at night by at least six hours to prevent a compound effect of the two medications.
Patients aged 65 years and over should be screened for hyponatraemia after one week of desmopressin/furosemide therapy and again at one and six months or if there is a change in medication or health status. Antidiuresis therapy should be stopped if sodium levels dip below 130 mmol/L; a cause for this drop should be investigated (eg concomitant lithium, thiazides, carbamazepine or oedema) to determine the best means of intervention.
Conclusion
Nocturia twice or more per night is rarely a benign symptom. Specific questions that will identify clinically relevant comorbidities that may be inducing a mismatch between urine production and bladder storage have been presented. Treatment strategies can be individualised to target these factors.
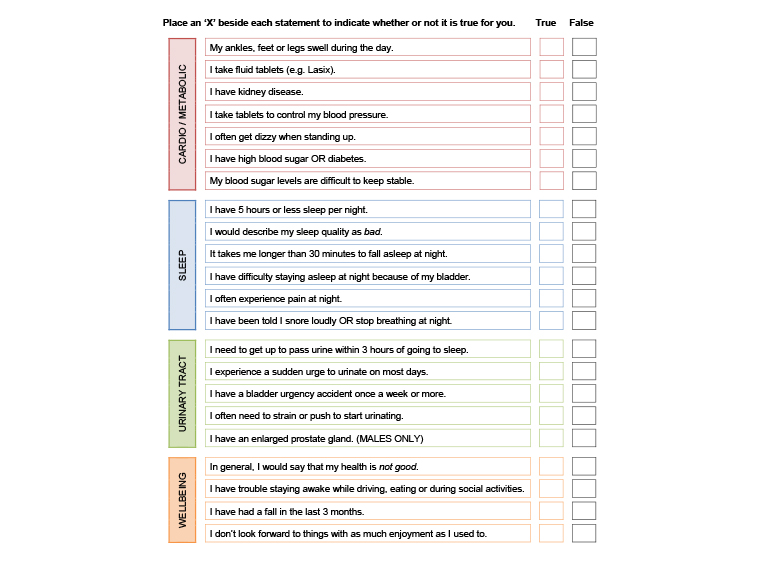
Appendix 1. TANGO nocturia screen
Reproduced with permission from Australian New Zealand Continence Journal, from Rose GE, Bower WF, Ervin CF, Whishaw DM, Khan F. Reliability Testing of the TANGO Short-Form nocturia screening. Aust N Z Cont J 2017;23(3):68–74.