This paper provides a summary of the three most recent international, evidence-based guidelines on recurrent pregnancy loss (RPL) – the Royal College of Obstetricians and Gynaecologists (RCOG) 2011 guidelines on recurrent miscarriage,1 the American Society for Reproductive Medicine (ASRM) 2012 RPL guidelines,2 and the European Society of Human Reproduction and Embryology (ESHRE) 2017 RPL guidelines.3
Pregnancy loss is defined as the loss of a pregnancy prior to 24 weeks gestation. RPL has previously been defined as three or more pregnancy losses.1 This affects 1% of couples. However, more recent guidelines have amended this definition to two or more pregnancy losses.2,3 This change has occurred because of a combination of patient distress and an insignificant change in positive investigation results between two and three pregnancy losses. Whether the losses are consecutive or non-consecutive does not seem to affect the aetiology, investigation or management of RPL.3
RPL takes a significant emotional toll on women and their partners. For many couples, miscarriage represents the loss of a child along with their hopes for that child. It is therefore common for patients to experience loss and grief, and it is not surprising that women and their partners are anxious in subsequent pregnancies. Management of these couples in an organised multidisciplinary team setting is recommended.3
Recommendation
Couples should be referred to a specialised multidisciplinary clinic after two pregnancy losses.
Epidemiology
The incidence of pregnancy loss among clinically recognised pregnancies is 12–15%.3 However, unrecognised pregnancy loss is thought to be much greater – there is suggestion that 15% of fertilised ova are lost before implantation, with an overall conception loss rate of up to 52%.3,4
Maternal age and number of previous miscarriages independently predict future miscarriage (Table 1). One per cent of couples will experience three or more losses;1 5% will experience two or more losses.2 The ESHRE guidelines emphasise the need to have at least a positive b-human chorionic gonadotropin (b-hCG) level to confirm pregnancy.5
|
Table 1. Pregnancy loss by maternal age1
|
|
Maternal age (years)
|
Rate of pregnancy loss (%)
|
|
20–24
|
11
|
|
25–29
|
12
|
|
30–34
|
15
|
|
35–39
|
25
|
|
40–44
|
51
|
|
>45
|
93
|
Aetiology, investigations and management
Genetic causes
In 2–5% of couples with RPL, one of the partners carries a balanced chromosomal anomaly.1 These carriers are phenotypically normal but their pregnancies are at increased risk of miscarriage or live births to offspring with congenital abnormalities.
The RCOG states that cytogenetic analysis should be performed on products of conception (POC) in patients with RPL.1 Peripheral blood karyotyping of both parents should be performed if the POC have an unbalanced structural chromosomal abnormality. However, the ASRM recommends that all RPL parents should have peripheral karyotyping independently of the POC karyotyping.
Where a karyotypic abnormality is detected, genetic counselling is warranted. Options for these couples include:
The ESHRE guidelines are more sceptical of the value of routine karyotyping of parents and POC. Karyotyping of the POC is not without issues (eg difficulty of obtaining tissue, incorrect preparation, maternal contamination and failed tests) but may be useful for explanatory purposes. Karyotyping of the parents is not routinely recommended because ongoing pregnancies (ie viable pregnancies over 20 weeks gestation) with unbalanced translocations in carrier parents are very rare (<1%). Furthermore, the long-term cumulative live birth rates in carriers of chromosomal abnormalities are good (71% in two years). Additionally, once identified, 15% of carrier couples opt to not try again. Therefore, it is possible that identification of a carrier may have a negative impact on future pregnancy rates.3
Clearly, this is a controversial area. Currently, no Australian RPL guidelines exist. However, a guideline by the Australasian CREI Consensus Expert Panel on Trial evidence (ACCEPT) group is in development. Preliminary discussions suggest that karyotyping of POC and parents is valuable because of availability and access to PGD in Australia.
Recommendation
Karyotyping of parents and POC is recommended for couples with two or more pregnancy losses.
Anatomical causes
Many anatomical anomalies have been associated with RPL. Uterine leiomyoma, Müllerian anomalies and uterine synechiae are the most significant.
Leiomyoma
Fibroids are common and can be categorised as subserosal (serosal component), intramural (predominant myometrial component) and submucosal (intracavity component).6,7
Combination hysteroscopy and laparoscopy remain the gold standard for diagnosing uterine pathology. However, it is agreed that sonohysterography or hysterosalpingography are effective and less invasive ways of diagnosing uterine pathology.8
The 2011 ACCEPT guidelines (reviewed in 2016) suggest that subserosal fibroids have no impact on fertility or miscarriage, intramural fibroids may decrease live birth and increase miscarriage rates, and submucosal fibroids decrease live birth rates and increase miscarriage rates. ACCEPT recommends that submucosal fibroids be removed in women to improve pregnancy outcomes. They also suggest that the evidence for removing intramural fibroids is uncertain.6
The RCOG guidelines are silent on the role of fibroids in miscarriage. The ESHRE guidelines note that the role of fibroids is controversial, but surgical management can be considered on a case-by-case basis.
Müllerian anomalies
The RCOG guidelines note that the rate of Müllerian anomalies in those with RPL varies from 1.5% to 37%.1 The ASRM guidelines state that the rate of Müllerian anomalies is about 4% in women without RPL, whereas it is 12.6% in those with RPL.2 There is also an association between Müllerian anomalies and second trimester loss. There is some evidence that the septate uterus is associated with a higher rate of pregnancy loss and that correction can lead to reduced rates of miscarriage and thus should be considered in those with RPL.2 The correction of other Müllerian anomalies is not associated with any improvement in miscarriage rates.
The ESHRE guidelines recognise that the septate uterus is linked to first trimester loss but note that the evidence for treatment and subsequent reduction in incidence of miscarriage is weak. It recommends that surgical treatment of septa be attempted in the context of a clinical trial.
Uterine synechiae
Uterine synechiae (Asherman’s syndrome) may be asymptomatic. However, menstrual cycle disturbances, specifically hypomenorrhoea and dysmenorrhea, are common. Synechiae may also cause infertility, and there is some evidence that they increase the chance of miscarriage.9
The RCOG does not mention uterine synechiae in their guidelines. The ASRM guidelines recognise that their link to RPL is controversial but also understand that randomised controlled trials (RCTs) are difficult to perform in this context. Thus it recommends correction of synechiae in RPL after discussion with patients.2 The ESHRE guidelines point out that there is weak evidence for resection of uterine synechiae in reducing miscarriage rates but note that the surgery itself can promote more adhesion formation, so precautions must be taken in the perioperative setting to minimise their formation.3
Recommendation
Two-dimensional/three-dimensional ultrasonography with sonohysterography is recommended for couples with two or more pregnancy losses.
Thrombophilia
Congenital thrombophilia
Congenital thrombophilias (Factor V Leiden, prothrombin gene mutation and deficiencies in anti-thrombin, protein C and protein S) all increase a woman’s chance of developing thromboembolism. They may also be associated with adverse pregnancy outcomes.
However, the evidence linking these thrombophilias to RPL is based on weak studies and is inconclusive. As a result, no guidelines recommend investigating for congenital thrombophilias outside of a research setting.10
Acquired thrombophilias
Antiphospholipid syndrome (APS) and its associated antibodies (anti-cardiolipin and lupus anticoagulant antibodies) are linked to RPL.11 Possible mechanisms include direct inhibition of placentation, disruption of adhesion molecules and thrombosis of placental vasculature. All three guidelines suggest testing for APS in RPL. There is also some evidence linking RPL to b2 glycoprotein1 (b2GP1) antibodies; thus, both the ASRM and ESHRE guidelines suggest including b2GP1 antibodies in the investigations.
Evidence for treatment of acquired thrombophilias exists in the context of a diagnosis of APS. In those with RPL and APS, the combination of 75–100 mg aspirin daily with prophylactic doses of unfractionated heparin has been shown to significantly reduce the rate of miscarriage. Aspirin alone seems to be ineffective.11
Recommendations
For couples with two or more pregnancy losses:
Endocrinological causes
Endocrinological associations investigated in the context of RPL include thyroid function, glucose metabolism, polycystic ovary syndrome (PCOS), progesterone and prolactin.
There is evidence that suggests hypothyroidism – and even subclinical hypothyroidism – is associated with RPL.2,3 All guidelines recommend testing for thyroid-stimulating hormone (TSH) levels, but there is contention about what is considered a ‘normal’ TSH. Furthermore, there is controversy regarding the significance of thyroid antibodies, especially in the context of a ‘normal’ TSH. Current guidelines suggest treating all women with overt hypothyroidism, considering treatment of subclinical hypothyroidism, and not treating euthyroid patients with RPL who test positive for thyroid antibodies.12
All guidelines suggest that well‑controlled diabetes is not a risk factor for RPL, but poorly controlled diabetes is. Routine screening for PCOS is not recommended for treatment or investigation of RPL.13 There is no evidence for the use of metformin to prevent RPL.14
Elevated prolactin may cause ovulatory dysfunction. However, the link with RPL is tenuous. The ESHRE guidelines do not recommend testing prolactin in the absence of clinical suspicion; ASRM states that testing can be considered. There is some weak evidence to suggest that normalising hyperprolactinaemia with a dopamine agonist can improve live births in RPL. The agent with most evidence is bromocriptine.3
There is conflicting evidence regarding the use of progesterone in RPL. A Cochrane meta-analysis concluded that progesterone does reduce further miscarriage in women with RPL. However, only small, underpowered studies were included.15 The largest RCT (not included in the Cochrane meta-analysis) failed to demonstrate a benefit. As such, all guidelines recommend against using progesterone in RPL, but it is noted that progesterone supplementation causes no harm.2,3,16
Recommendation
In couples with two or more pregnancy losses:
-
thyroid function tests and antibodies should be performed.
-
the role of abnormal prolactin levels, PCOS and progesterone supplementation is uncertain.
Infection
While any overwhelming infection will cause miscarriage, there is no clear link between chronic infection and RPL. There is evidence that bacterial vaginosis can lead to miscarriage in the second trimester; the evidence for its link to first trimester miscarriage is tenuous. Work done in Ureaplasma, Listeria, Chlamydia and Mycoplasma, and toxoplasmosis, other agents, rubella, Cytomegalovirus and Herpes simplex (TORCH) infections has not shown any link to RPL. It must also be noted that there is a general paucity in prospective studies in this area.3
Immune system
There has been much recent interest in the field of immunology and its relationship to RPL. This includes the study of human leukocyte antigen (HLA) typing, natural killer cells and immunomodulation with, for example, intravenous immunoglobulin, corticosteroids, HLA modification, intralipid infusion, auto-transfusion of lymphocytes and platelet rich plasma. A full discussion of immunotherapy is beyond the scope of this article, but there is currently no good evidence that immunomodulation has any effect on RPL. Investigations for auto-immunity outside of APS are not recommended.3
Environment and lifestyle
Most data looking at environmental effects focus on sporadic miscarriage rather than RPL. However, cigarette smoking is linked to increased rates of miscarriage due to trophoblastic dysfunction. Alcohol and caffeine intake increase the risk of miscarriage in a dose-dependent manner.17 Illicit drug use, especially cocaine use, leads to an increased risk of miscarriage. Stress is not a direct cause of RPL. Female obesity is linked to increased miscarriage rates and can cause other pregnancy-related complications.18
All guidelines recommend ceasing smoking and alcohol consumption, limiting caffeine intake to fewer than three cups of coffee per day and normalising body mass index (BMI). While stress is not a direct cause of RPL, there is evidence that care in a specialised clinic that provides a supportive environment does decrease the chance of miscarriage and increases live birth.1
Male factors
Lifestyle factors such as normalisation of BMI, cessation of smoking and reduction of alcohol intake are recommended by all guidelines.
Semen analysis by itself does not seem to be predictive of miscarriage. However, there is conflicting evidence about the significance of high sperm DNA fragmentation. Some studies suggest that DNA fragmentation is increased with RPL, especially in the in vitro fertilisation setting.19,20 The ASRM guidelines state that routine sperm DNA fragmentation indexing is not indicated because of the weak evidence, but the ESHRE guidelines state that this can be done to provide an explanation for RPL.
Recommendation
For couples with two or more pregnancy losses, both partners should:
-
cease smoking and alcohol consumption
-
cease illicit substance use
-
limit caffeine consumption to three or fewer cups per day
-
normalise BMI.
Unexplained recurrent pregnancy loss
RPL remains unexplained in up to 50–75% of cases. This can be difficult for couples to accept, and care in a multidisciplinary, specialised unit is paramount and has been shown to lead to better outcomes. A couple’s chance of a successful pregnancy depends on age and previous parity, but can be beyond 50–60%.1
Conclusion
RPL is defined as two or more pregnancy losses. It affects <5% of couples. Tables 2 and 3 outline investigations and management respectively, while Figure 1 summarises an approach to management. A significant proportion of couples presenting with RPL will not have a cause identified. It is thus important that all couples are offered referrals to centres or clinicians with specific expertise in the management and counselling of this condition.
|
Table 2. Investigation summary for recurrent pregnancy loss
|
|
Investigations
|
Yes
|
Maybe
|
No
|
|
Anatomical
|
Two-dimensional/three-dimensional ultrasonography and sonohysterography or
Combination laparosopy and hysteroscopy
|
MRI
|
|
|
Genetic
|
Karyotype: POC
|
Karyotype: parental
|
|
|
Thrombophilia
|
Acquired: APS
|
Anti-b2 glycoprotein
|
Congenital thrombophilia
|
|
Infection
|
|
LVS/HVS/chlamydia
Endometrial biopsy and culture
|
TORCH
|
|
Immunological
|
|
Antinuclear antibody
|
HLA
Natural killer cells (research only)
|
|
Endocrinological
|
TSH (FT3/4 and antibodies if TSH abnormal)
|
Prolactin
|
|
|
Male factor
|
|
Sperm DNA fragmentation index
|
|
|
APS, antiphospholipid syndrome; DNA, deoxyribonucleic acid; FT3, free triiodothyronine; FT4, free thyroxine; HLA, human leukocyte antigen; HVS, high vaginal swab; LVS, low vaginal swab; MRI, magnetic resonance imaging; POC, products of conception; TORCH, toxoplasmosis, other agents, rubella, Cytomegalovirus and Herpes simplex; TSH, thyroid-stimulating hormone
|
|
Table 3. Treatment summary for recurrent pregnancy loss
|
|
Treatment
|
Yes
|
Maybe
|
No
|
|
Anatomical
|
Submucosal fibroid surgical management suggested
|
Uterine septa
Endometrial polyps
Uterine synechiae
|
Other Müllerian anomalies
|
|
Genetic
|
Pre-implantation genetic diagnosis (in known parental karyotypic abnormalities)
|
Pre-implantation genetic screening
|
|
|
Thrombophilia
|
Aspirin and unfractionated heparin in the context of APS
|
|
Aspirin
|
|
Infection
|
Antibiotics: if clinical evidence of infection
|
|
Prophylactic antibiotics
|
|
Immunological
|
|
|
Prednisone
IVIG
Partner lymphocyte transfusion
Intralipid
|
|
Endocrinological
|
Control of diabetes mellitus
Overt hypo/hyperthyroidism
|
Subclinical hypothyroidism
Progesterone
|
Androgens
β-hCG
LH
|
|
Male factor
|
Lifestyle modification
|
PICSI
IMSI
Antioxidants
|
|
|
Environment/lifestyle
|
Smoking: cease
Illicit drugs: cease
Maintain normal BMI
Specialised and individualised care in dedicated clinic
|
Limiting caffeine to
≤3 serves per day
|
|
|
APS, Antiphospholipid syndrome; β-hCG, beta human chorionic gonadotropin; BMI, body mass index; IMSI, intracytoplasmic morphologically selected sperm injection; IVIG, intravenous immunoglobulin; LH, luteinising hormone; PICSI, physiological intracytoplasmic sperm injection; TORCH, toxoplasmosis, other agents, rubella, Cytomegalovirus and Herpes simplex
|
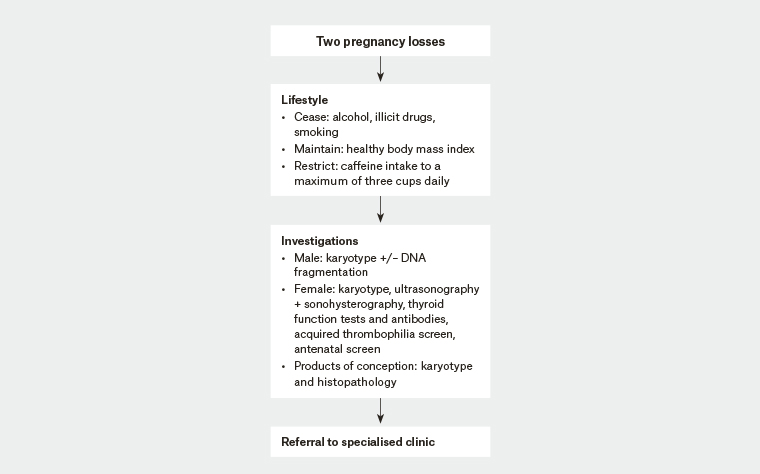
Figure 1. Recurrent pregnancy loss management summary