Leptospirosis is caused by bacteria of the genus
Leptospira, which has >250 known pathogenic serovars.
1 Leptospira colonise the kidneys of infected mammals and are released through urine into the environment, where they can survive for weeks. Humans become infected through direct contact with infected animals, including rodents, wildlife, livestock, and pets, or contact with soil or water contaminated by the urine of infected animals. Risk factors for infection depend on interactions between humans, animals and the environment (Figure 1).
Globally, leptospirosis is an emerging infectious disease with a rising incidence, increasing frequency and severity of outbreaks, and evolving climatic, sociodemographic and environmental drivers of transmission.2 Recently, unprecedented outbreaks have occurred as a result of the combined forces of climate change, flooding, population growth, urbanisation (typically associated with poverty and slums in developing countries), and agricultural intensification to meet the increasing demands for food.3
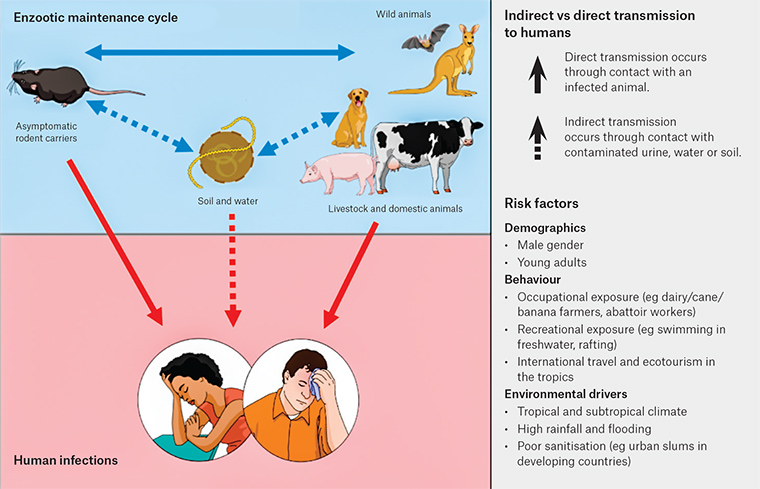
Figure 1. Transmission pathways and risk factors for leptospirosis
Epidemiology and risk factors
Leptospirosis is responsible for about one million severe cases and 60,000 deaths per year worldwide.4 The incidence of leptospirosis is highest in tropical and subtropical areas, and disease burden is particularly high in Oceania.5 Summary statistics of cases reported to the National Notifiable Disease Surveillance System (NNDSS) in Australia are shown in Figure 2. The average notification rate from 2007 to 2016 was 0.55 cases per 100,000 population per year,6 ranging from 1.85 cases per 100,000 population per year in Queensland, to 0.94 cases per 100,000 population per year in the Northern Territory, and <0.3 cases per 100,000 population per year in the other states and territories. Queensland had the greatest infection burden, particularly in the Cairns and Hinterland region,7 and the spike in cases in 2011 was attributed to an outbreak after unprecedented flooding across Queensland.8,9 Most cases occurred in the summer months, and in young and middle-aged males.6
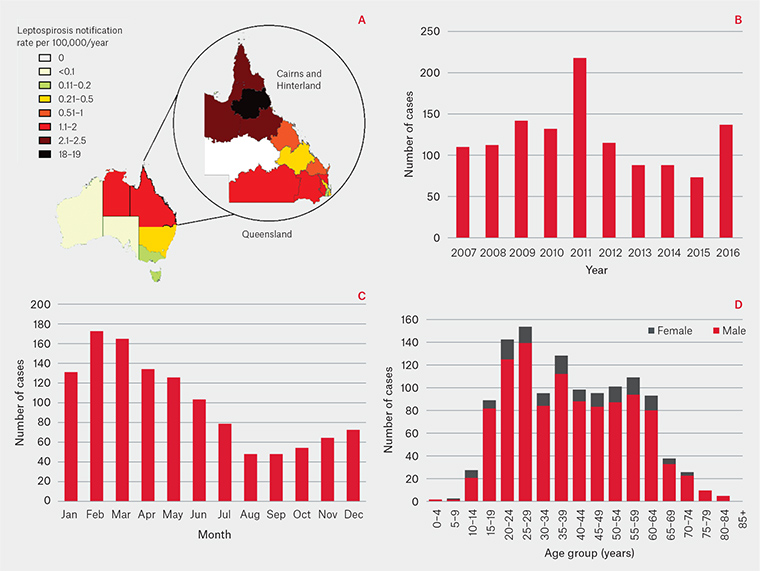
Figure 2. Leptospirosis cases notified to NNDSS and Queensland Health: (A) incidence by Australian states and territories6 and Queensland Hospital and Health Services areas7 from 2012 to 2016; (B) cases per year; (C) cases by month; (D) cases by age and sex from 2007 to 20166
In Australia, occupational exposure is the predominant source of infection, particularly in livestock and dairy farmers, abattoir and meat workers, and banana workers (through exposure to rodents).10 Infection through recreational exposure is also common, mostly from white water rafting, kayaking, ecotourism and other outdoor activities.11 International travel, particularly to tropical, developing countries, is an important source of occupational and recreational exposure.11
In tropical, developing countries, including Australia’s neighbours in the Asia Pacific region, environmental and climatic drivers are important. Tropical climates provide ideal conditions for Leptospira to survive, and transmission is exacerbated during high rainfall and flooding, when bacteria are widely disseminated.3 Exposure is even more intense in places with poor sanitation, inadequate drainage and abundance of rodents (eg urban slums). Many post-flooding outbreaks have therefore occurred in densely populated developing countries, including Brazil,12 India13 and the Philippines.14
Pathophysiology, clinical features and complications
Clinical presentations of leptospirosis range from mild, non-specific febrile illnesses to life-threatening complications. After an incubation period of two to 30 days (usually five to 14 days), leptospirosis manifests as a biphasic illness with an acute/bacteraemic phase (seven to 10 days from symptom onset), characterised by sudden onset of fever, myalgia and headache.2 Calf tenderness and conjunctival suffusion are characteristic but not always present. Other non-specific symptoms include anorexia, nausea, vomiting, abdominal pain, dizziness, lethargy, arthralgia, eye pain, photophobia and rashes. This is followed by a late/immune phase (>7 days from symptom onset), when immunologically mediated organ damage occurs, and severe complications include acute renal failure, pulmonary haemorrhage, myocarditis, arrhythmias, shock, liver failure, coagulopathy and neurological complications.2 Weil’s disease is the classic triad of jaundice, renal failure and haemorrhage, but these manifestations do not always occur together. Aseptic meningitis, encephalitis, convulsions, Guillain–Barré syndrome, transverse myelitis and other neurological syndromes have also been reported.2 Leptospirosis can also present with acute or chronic optic manifestations, including subconjunctival and retinal haemorrhages, optic neuritis and chronic uveitis.
Differential diagnosis
Leptospirosis can be difficult to clinically distinguish from other causes of acute febrile illnesses or their severe complications; diagnosis may therefore be missed or delayed. A detailed history is important for identifying patients who have undertaken activities that place them at risk of exposure to infection. A travel history is also important for delineating possible diagnoses.
Differential diagnoses of leptospirosis include influenza, pneumonia, arboviral infections (ie dengue, chikungunya, Zika virus, Ross River virus), malaria, typhoid, rickettsia, Q fever, acute viral hepatitis, pyelonephritis and meningitis. Haemorrhagic complications can also mimic meningococcaemia or severe dengue.15 Co-infections have also been reported,16 particularly in post-flooding settings, when outbreaks of multiple diseases (eg leptospirosis, dengue, typhoid) can occur at the same time.
Laboratory diagnosis
Multiple diagnostic tests are available, and it is important to order the appropriate test(s) and understand their interpretation for each phase of the illness. Tests that detect the presence of bacteria (eg polymerase chain reaction [PCR], culture) will only yield positive results in the acute/bacteraemic phase. Tests that detect antibodies will produce positive results later, from days six to eight from illness onset for immunoglobulin M (IgM) enzyme-linked immunosorbent assay (ELISA), and from day 10 to 12 from illness onset for microscopic agglutination test (MAT). The NNDSS definition of a confirmed case (Box 1) is based on culture or MAT, the gold standard tests in the acute/bacteraemic and late/immune phases respectively.17 Positive PCR alone or IgM ELISA alone are considered probable rather than confirmatory, but are nevertheless valuable for guiding clinical diagnosis in patients who present with suspected leptospirosis.
| Box 1. Case definition of leptospirosis17 |
A ‘confirmed case’ requires definitive laboratory evidence of leptospirosis infection by one of the following:
- Isolation of pathogenic Leptospira species
- A fourfold or greater rise in Leptospira MAT titre between acute-and convalescent-phase sera obtained at least two weeks apart, and preferably conducted at the same laboratory
- A single Leptospira MAT titre ≥400, supported by a positive ELISA IgM result.
|
Note. A positive IgM ELISA or positive PCR alone are considered probable rather than confirmatory according to the National Notifiable Diseases Surveillance System definitions
ELISA, enzyme-linked immunosorbent assay; MAT, microscopic agglutination test; PCR, polymerase chain reaction |
In the acute/bacteraemic phase, blood should be collected for PCR (in a serum-separating tube) and IgM ELISA before commencing antibiotics. During the early acute/bacteraemic phase, IgM ELISA has lower sensitivity, compared with PCR,18 but the results are helpful in determining the phase of illness. Blood cultures should be taken if the specific culture medium is available (Ellinghausen–McCullough–Johnson–Harris medium).19 However, it is important to check the availability of the culture medium with the local pathology laboratory, and whether they are able to conduct cultures. Cultures are examined for six weeks by dark-field microscopy, so this method is generally not useful for informing immediate clinical management.
During the late/immune phase, when antibodies are present, both IgM ELISA and MAT tests should be requested. Reactive IgM ELISAs are sent to reference laboratories for confirmation by MAT. A convalescent sample should be collected 14 days later to confirm rising MAT titres, particularly if the initial IgM ELISA was reactive but MAT was found to be non-reactive. MAT combines diluted serum with a panel of serovars from different serogroups.1,19 The World Health Organization’s (WHO’s) Leptospirosis Reference Laboratory in Brisbane, Queensland,20 uses a routine panel of 22 serovars, which includes a representative from each major serogroup. Any history of overseas travel by the patient should be communicated to the laboratory so that appropriate serovars are included in the panel.
Depending on the clinical presentation, other relevant investigations should include full blood count, biochemistry, arterial blood gases, electrocardiography (ECG), chest X-ray and lumbar puncture. The most common laboratory findings in a patient with leptospirosis are neutrophilia and mildly abnormal liver function tests. Other possible investigation findings, depending on severity and complications, are summarised in Table 1.
| Table 1. Possible investigation findings in leptospirosis, depending on severity and complications |
| Investigations |
Findings |
| Full blood count |
Leucocytosis, neutrophilia with left shift, lymphopenia, normochromic anaemia, thrombocytopenia |
| Urea, electrolytes, creatinine |
Raised urea and creatinine if renal impairment. Potassium is usually normal or low, high potassium is associated with poor outcomes (an indicator of impaired renal function, and might lead to arrhythmias). Low sodium |
| Liver function tests |
Raised bilirubin (mainly direct), may take time to resolve. Normal or raised liver enzymes. Aspartate aminotransferase and alanine aminotransferase are typically three to five times above normal, but could be much higher in cases with fulminant hepatic failure |
| Urinalysis |
Proteinuria, microscopic haematuria, pyuria, granular casts |
| Creatine phosphokinase |
Raised in patients with myalgia |
| Coagulation |
Prothrombin time, partial thromboplastin time and international normalised ratio may be raised because of impaired liver function |
| Arterial blood gases |
Low partial pressure of O2 (PaO2), arterial O2 saturation (SaO2), ratio of partial pressure arterial oxygen and fraction of inspired oxygen (PaO2/FiO2) ratio, metabolic acidosis (ie low pH, low HCO3) |
| Electrocardiograph |
Atrial fibrillation, supraventricular or ventricular extrasystoles, atrioventricular block, other arrhythmias |
| Chest X-ray |
Variable findings, including alveolar infiltrates, nodular densities and consolidation. Changes could be diffuse or lobar, unilateral or bilateral. Findings could represent a range of pathology, including alveolar haemorrhage, acute respiratory distress syndrome, pulmonary oedema |
| Lumbar puncture |
Neutrophilic or lymphocytic pleocytosis, mild elevations in protein, normal glucose |
Clinical management
If leptospirosis is suspected on the basis of the clinical presentation and risk factors for recent exposure, empirical antibiotics should be given early and continued for at least seven days. For mild disease, doxycycline (100 mg twice daily) is the preferred antibiotic for empirical treatment because it is also effective against rickettsial infections, which can have a similar presentation.21 Other options include amoxicillin and erythromycin. Intravenous benzylpenicillin 1.2 g six-hourly or ceftriaxone 1 g daily should be used for severe infection.21 Jarish–Herxheimer reactions can occur one to 48 hours after starting antibiotics.15,22 Common features include sudden onset of shivers or rigors, fever and hypotension. Jarish–Herxheimer is caused by an acute inflammatory response mediated by the release of large amounts of cytokines in response to the sudden and rapid death of Leptospira.
Patients with suspected leptospirosis who present with life-threatening complications and/or evidence of end-organ failure (eg massive pulmonary haemorrhage, acute respiratory distress syndrome, renal failure, shock) should be referred to a hospital because of the risk of further deterioration.
Depending on the complications, management might include intravenous fluids and inotropes, ventilation, haemodialysis, and treatment of arrhythmias and coagulopathies. Currently, corticosteroids are not recommended because there is conflicting evidence about their effectiveness, and their use has been associated with increased risk of nosocomial infections in patients with severe leptospirosis.23
Case
A mining engineer, 38 years of age, presented to a general practitioner with a one-week history of fevers, rigors, nausea, vomiting, headache, myalgia and malaise. He had returned two weeks previously from a one-month work trip to Papua New Guinea (PNG), where he walked in jungles and creeks, sustained cuts and lacerations, and received multiple insect bites. He drank filtered water, ate cooked or processed food, and denied any recent sexual contact. He was up to date with routine immunisations, and hepatitis A and typhoid immunisations. He had no significant medical history, and was not on any medications.
On examination, he was icteric, febrile (39.9
oC) and tachycardic (110 beats/min). His blood pressure was 140/80 mmHg, respiratory rate was 20 breaths/min and O
2 saturation was 98% on air. Cardiorespiratory examinations were unremarkable. Right upper quadrant abdominal tenderness and mild hepatomegaly were noted. There was no rash or lymphadenopathy. He was referred to an emergency department, where initial investigations revealed:
- normal haemoglobin – 163 g/L
- marked leukopenia – 2.7 x 109/L (reference range: 4.5–11 x 109/L)
- lymphopenia – 0.36 x 109/L (reference range: 1–4 x 109/L)
- thrombocytopenia – 93 x 109/L (reference range: 150–400 x 109/L)
- acute kidney injury –
- creatinine: 120 µmol/L
- estimated glomerular filtration rate: 46 mL/min/1.73 m2
- acute hepatitis –
- total serum protein: 61 g/L (reference range: 65–85 g/L)
- aspartate aminotransferase: 400 U/L (reference range: 10–45 U/L)
- alanine aminotransferase: 364 U/L (reference range: 5–45 U/L)
- bilirubin: 112 µmol/L (reference range: <20 µmol/L)
- elevated lactate – 3.4 mmol/L (reference range: <2 mmol/L)
- elevated C-reactive protein – 232 mg/L (reference range: <5 mg/L).
International normalised ratio and chest X-ray were normal. An abdominal ultrasound showed a mildly enlarged liver consistent with acute hepatitis, but was otherwise normal. Blood cultures and laboratory tests for malaria, dengue, human immunodeficiency virus, cytomegalovirus, Zika virus, flavivirus, rickettsia and hepatitis C were all negative. Test results also showed immunity to Epstein–Barr virus, and hepatitis A and B. Leptospira IgM ELISA was reactive, but MAT was negative (titre <1:50).
He was admitted to hospital and commenced on doxycycline 100 mg twice daily for presumed leptospirosis. Over three days, his fever and symptoms resolved, and blood markers improved. He was discharged home to complete a course of doxycycline, and made a full recovery.
Follow-up serology showed persistently reactive IgM ELISA, but only low MAT titres (1:100 against serovar Celledoni), suggestive, but not definitive, of recent infection. Leptospira DNA was detected by PCR performed retrospectively on the initial specimen. The inconclusive serology results most likely represent infection with an unusual or yet unidentified serovar from PNG.
This case illustrates a classic presentation of severe leptospirosis after high-risk exposure in a tropical, developing country, and the challenges of confirming infections with exotic serovars. The patient presented at one week after symptom onset (at the transition between acute/bacteraemic and late/immune phase), and a more timely diagnosis could have been made if both PCR and serology were initially requested.
Surveillance, prevention and control
Leptospirosis is a notifiable disease, and reporting to NNDSS is mandatory. Cases are investigated by the public health units of the specific state and territory, and risk factors and potential infection sources are explored.
Infection risk can be reduced by avoiding direct contact with contaminated soil or water (especially floodwaters), and animals or animal products. Those at occupational risk, such as farmers, abattoir and meat workers, banana workers and military personnel, should be provided with educational materials about preventive measures24 and advised to wear protective gear (eg boots, goggles, aprons, gloves). Open wounds should be cleaned and covered. Thorough washing is recommended after exposure to contaminated soil, water, animals or animal products. Travellers to tropical developing countries should be advised to avoid floodwaters, or swimming in rivers and waterfalls after heavy rainfall. Those at high risk of infection should be advised to seek medical care promptly if they develop a febrile illness.
Infection risk can also be reduced by control measures aimed at minimising environmental contamination, including livestock vaccination, segregating infected animals, rodent control, flood mitigation and reducing garbage, which attracts rodents and blocks drains. Sources of infection identified through case investigations should be managed appropriately to reduce the risk of further cases. During high-risk periods (eg post-flooding), public health warnings and alerts should be issued.
Doxycycline has been considered for prophylaxis, but a systematic review concluded that regular use at 200 mg weekly was associated with nausea and vomiting, and there was no clear benefit for reducing the risk of leptospirosis.25 Routine use is therefore not recommended, but might be considered if short-term intense exposures are anticipated (eg soldiers, outbreak response personnel, recreational exposure) or after high-risk exposures (eg floodwaters). Doxycycline can also provide protection against other infectious disease (eg malaria, rickettsia). For travellers to malaria-endemic areas who may be at risk of leptospirosis, using doxycycline for malaria prophylaxis (in preference to other antimalarial medications) could be considered. Human vaccines are rarely used (none are available in Australia) because of limited effectiveness, adverse reactions and short duration of protection.26
Key points
-
For patients with risk factors, leptospirosis should be considered as a differential diagnosis of undifferentiated febrile illness and the complications discussed above.
-
A high index of clinical suspicion is required to ensure early diagnosis and treatment, and to minimise the risk of complications.
-
Patients involved in high-risk activities should be advised to seek medical care promptly if they develop a fever.
-
Diagnosis is confirmed by serology or culture, but PCR is valuable in the early phase of the infection.
- Severe cases and complications should be referred to a hospital for inpatient management.
Resources
- eTG, Antibiotic, https://tgldcdp.tg.org.au/etgAccess
- WHO/FAO/OIE Leptospirosis Reference Laboratory, Health Support Queensland, www.health.qld.gov.au/qhcss/lepto
- Queensland Health’s guidelines for public health units, including laboratory diagnosis, case definitions, preventative measures, www.health.qld.gov.au/cdcg/index/lepto
- Prevention and control of leptospirosis (educational materials for farmers), http://saferfarms.org.nz/guides/prevention-and-control-of-leptospirosis
- World Health Organization, Leptospirosis, www.who.int/topics/leptospirosis/en
- US Centres for Disease Control and Prevention, Leptospirosis, www.cdc.gov/leptospirosis