Cardiac rhythm management devices include pacemakers, implantable cardioverter defibrillators (ICDs) and loop recorders (Table 1). Australia has an impressive history with respect to cardiac pacing. The world’s first artificial pacemaker was created by Australian Dr Mark Lidwill, an anaesthetist at Royal Prince Alfred Hospital, in 1926.1 It took another three decades, however, before the first implantable pacemaker was inserted, in Sweden in 1958.1 Within three years of this, the first Australian implant insertion was performed at the Royal Melbourne Hospital.2 Around 20,000 cardiac rhythm management devices are implanted across Australia each year, approximately three-quarters of which are pacemakers.3
Therapies deliverable by cardiac rhythm management devices
Therapies deliverable by cardiac rhythm management devices are:
- cardiac resynchronisation therapy (CRT)/biventricular pacing
– Some patients with heart failure and reduced left ventricular function have poorly coordinated ventricular contraction. Biventricular pacing (pacing both the left and the right ventricles of the heart simultaneously) can improve systolic function by resynchronising the heart contraction (Figure 1).
– CRT is not indicated in all patients with heart failure. Those with a broad left bundle branch block and an ejection fraction ≤35% benefit the most.4
– CRT reduces cardiac failure symptoms, hospitalisation and mortality.5
-
defibrillation
– An electric shock is delivered to restore normal heart rhythm in the event of life-threatening rapid ventricular arrhythmias (ventricular fibrillation/fast ventricular tachycardia).
-
monitoring for heart rhythm disturbances
– Bradycardia, tachycardia, pauses, atrial fibrillation.
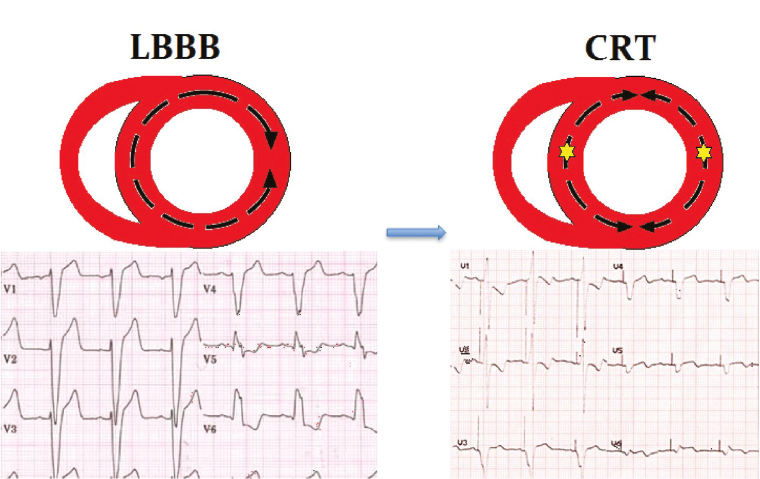
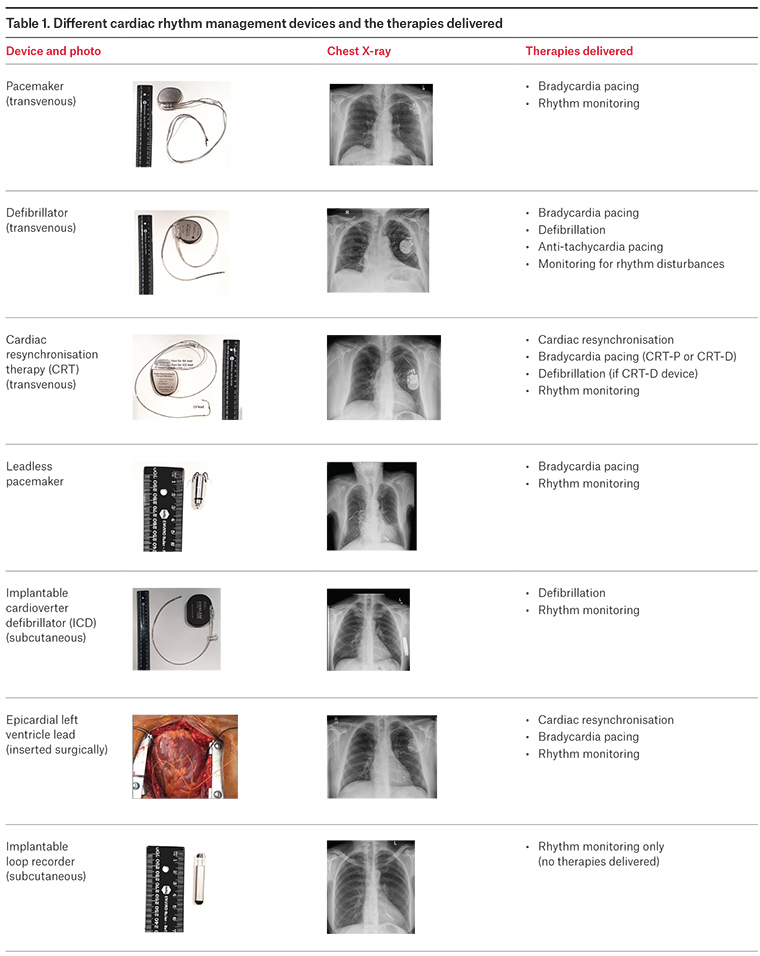
Figure 1. How cardiac resynchronisation therapy (CRT) works
The presence of a left bundle branch block creates ‘dyssynchrony’ as the septal wall is activated well ahead of the lateral wall. This is counteracted by CRT, which paces the septal and lateral walls together, thereby improving cardiac output
Reproduced with permission from Wiley Publishing Asia Pty Ltd, from Voskoboinik A, Ihle JF, Bloom JE, Kaye DM. Methamphetamine-associated cardiomyopathy: Patterns and predictors of recovery. Intern Med J 2016;46(6):723–27. doi: 10.1111/imj.13050.
Types of cardiac rhythm management devices
Pacemakers
Pacemakers send small electrical impulses to stimulate the heart to beat. They are usually implanted in patients with symptomatic bradycardia (most commonly sick sinus syndrome, atrioventricular [AV] block) – symptoms include presyncope, syncope, lethargy or exertional dyspnoea. They may also be implanted to facilitate medications that slow the heart rate4 – for instance, in patients with intermittent atrial fibrillation or those with heart failure that require beta blockers to improve their cardiac function.
Some patients may be entirely dependent on their pacemakers and have ‘no underlying rhythm’, while others may only pace intermittently (where the pacemaker is in place as a back-up). The chamber of the heart where the pacing electrodes sit may also vary:
- single lead (single chamber) – in the right ventricle or right atrium/transvenous or epicardial
- two leads (dual chamber) – in the right atrium and right ventricle/transvenous or epicardial
- three leads – in the right atrium, right ventricle and left ventricle/transvenous or epicardial
- leadless pacemaker – direct implant into the right ventricle.
Depending on the indication and the patient’s underlying rhythm, the pacemaker may be programmed in different ways. The nomenclature uses three to five letters. The first letter
refers to the chamber paced (A – atrium, V – ventricle, D – dual), the second to the chamber sensed (A – atrium, V – ventricle, D – dual), and the third to the response to a sensed event (O – none, I – inhibit, T – trigger, D – dual [ie trigger and inhibit]). A fourth letter (R) is often used if the pacemaker has rate-modulation capability, whereby the pacemaker increases the patient’s heart rate with exercise. A fifth letter relates to the presence and location of multi-site pacing, but is rarely used. Commonly programmed modes include:
- AAI: commonly used for patients with sinoatrial (SA) node dysfunction, the atrium is paced and sensed. If no atrial electrical impulse is sensed, the pacemaker will pace at a pre-programmed rate; if an electrical impulse is sensed, pacing is inhibited.
- VVI: commonly used for patients with slow atrial fibrillation, the ventricle is paced and sensed. If no ventricular impulse is sensed, the pacemaker will pace at a pre-programmed rate (often 60 beats per minute [bpm] – VVI 60). If an electrical impulse is sensed, pacing is inhibited.
- DDD: commonly used for patients with AV nodal disease (eg complete heart block). Both the atrium and ventricle are sensed and paced. If both the SA and AV node are functioning well, the pacemaker will simply sense the patient’s intrinsic rhythm. If no intrinsic impulses from the atrium or ventricle are detected, the pacemaker will take over (Figure 2).
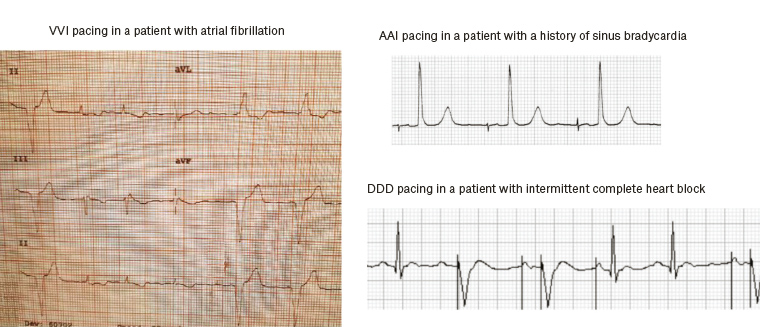 Figure 2. Electrocardiogram appearance of different pacing
Figure 2. Electrocardiogram appearance of different pacing
Defibrillators
Defibrillators are implanted in patients at risk of sudden cardiac death, and include patients who have reduced left ventricular function (often when ejection fraction is <35%) and cardiac arrest survivors:4
- single lead (single chamber) – in right ventricle
- two leads (dual chamber) – in right atrium and right ventricle
- three leads – in right atrium, right ventricle and left ventricle
- subcutaneous – no transvenous component.
Loop recorders
Loop monitors are implanted as a diagnostic tool and are only capable of monitoring for cardiac arrhythmias. They do not provide any therapies. Most commonly they are implanted to investigate unexplained syncope and, occasionally, palpitations. There is an emerging indication in patients with cryptogenic stroke, looking for atrial fibrillation as a cause.
Pre-operative considerations and implant procedure
Devices are usually implanted under conscious sedation as day case surgery or with an overnight stay. Blood-thinning medications may need to be withheld prior to device implantation, and this should be discussed with the implanting cardiologist. If a patient has an active infection at the time of planned device implant, the procedure may be deferred to prevent seeding of infection to the device. Patients are usually advised to fast for six hours prior to the procedure.
Traditional pacemakers can be on either the left or the right in the subclavicular region. Leads are inserted into the subclavian vein and guided into the heart chambers before being connected to the pulse generator. The pulse generator is positioned in a ‘pocket’, often between the subcutaneous tissue and the pectoral muscle, but sometimes deeper intramuscular or submuscular. The new leadless pacemakers are tiny 2 mL units implanted directly into the right ventricular apical region via a catheter from the groin.
ICDs are preferably positioned on the left because this is the best vector for defibrillation (from the lead in the right ventricle across the left ventricle to the pulse generator). The subcutaneous ICD pulse generator is placed in the mid axillary line, with the electrode tunnelled across the anterior chest wall and up along the sternum. The whole system remains on the outside of the thoracic cage, eliminating the risks associated with transvenous leads (endocarditis, tricuspid regurgitation, perforation, pneumothorax).
Loop recorders are placed subcutaneously on the left chest wall overlying the heart, usually around the level of the fourth intercostal space.
Post-procedural care
Patients with transvenous devices are advised to limit shoulder activity on the side ipsilateral to the device to reduce the risk of lead dislodgement. They should avoid lifting the ipsilateral elbow above shoulder height or lifting anything heavier than approximately 5 kg for two weeks post-implant. More strenuous activities such as golf and tennis should be delayed for approximately 4–6 weeks. Although positioned close to the shoulder, transvenous devices have no involvement of the joint itself and thus it is important to maintain gentle movement of the shoulder during this time to prevent a frozen shoulder. A sling should never be used.
Complications
Infections
- ·Risk of infection is lowest with the primary implant and increases with more complex procedures and every subsequent procedure.6
- Typically, infections are not evident until two to four weeks after the procedure.
- Infections may present as a local pocket infection with tenderness, erythema and discharge from the pocket or with systemic sepsis.
- Usually, systemic antibiotic therapy and device extraction is required.
Pocket haematoma
- Usually, these can be managed conservatively with pressure bandaging and adjustment of medications (ie anticoagulants) and rarely require evacuation.
- Pocket haematomas should never be aspirated with needles as this may introduce infection.
Lead perforation
- While this complication usually occurs acutely and may present with cardiac tamponade in hospital, a late presentation with a pericardial effusion may develop over the coming weeks and may present with symptoms of chest pain, fatigue and exertional dyspnoea.
- Clinicians should maintain a high index of suspicion, and referral for an echocardiogram should be considered.
Lead malfunction
- Lead-related problems may occur at any stage following implant and include dislodgement, fracture or breach of insulation.
- These may prevent the pacemaker from functioning appropriately, and patients may present with the same problems that necessitated implantation of the device in the first place, including dizziness and syncope.
- Patients should be referred for a device interrogation if there is any concern regarding pacemaker malfunction.
Diaphragmatic stimulation
- Diaphragmatic stimulation due to phrenic nerve capture is most commonly seen in patients with left ventricular leads (as part of cardiac resynchronisation therapy), and can usually be alleviated by device reprogramming.
Vein thrombosis
- Development of ipsilateral arm swelling in the weeks following pacemaker implantation should prompt consideration of deep venous thrombosis and referral for ultrasoography.
Significant adverse events may be reported to the Therapeutic Goods Administration website (www.tga.gov.au/reporting-adverse-events).
Routine follow-up
Pacemaker and defibrillator batteries can last for 5–15 years (depending on how often there are used, and the make and model). The generator needs to be changed when the battery is reaching the end of its life (this is critical in patients who are dependent on their pacemaker). Pacing leads remain in place indefinitely, but issues may develop over time. Hence, the pacemaker needs to be checked (interrogated) periodically. This is usually performed six weeks after implant, then every 6–12 months.
Remote monitoring
Remote monitoring from home is available with most contemporary devices. Some have wireless capability, whereas some require manual transmission using a handheld ‘wand’. Currently in Australia, only a fraction of patients are remotely monitored.7 Remote monitoring may reduce the number of in-person visits a patient requires. Remote monitoring may also detect device issues (eg battery or lead issues) and patient issues (eg new‑onset atrial fibrillation or ventricular arrhythmias). Remote monitoring has been shown to improve survival in patients with ICD and pacemaker.8,9 No programming changes are possible remotely.
Living with an implantable cardiac rhythm management device
Antibiotic prophylaxis
Antibiotic prophylaxis is not required for any medical, surgical or dental procedures in patients with implantable cardiac devices.10
Driving
Regulations vary from state to state in Australia and around the world, and there are specific exceptions and restrictions for private versus commercial licences. The Austroads Assessing fitness to drive should be consulted in specific cases.11 General guidelines are as follows:
- No driving for two weeks following primary prevention ICD, pacemaker implant or generator change.
- Patients with ICD implanted following cardiac arrest usually require a six-month event-free period before driving again.
- Patients with an ICD are not permitted to hold a commercial driving licence in Australia.
Travel
Manufacturers of cardiac rhythm devices implanted in Australia have representatives worldwide. All patients have an Implant Identification Card (Figure 3), which should be carried at all times and is useful when presenting at security checkpoints in airports or at emergency departments in hospital. This carries important information regarding the make and model of the device, leads present and contact details about who to call if there are any issues. The implanting cardiologist should be the first contact if there is concern regarding device malfunction.
Flying is generally safe 48 hours after an uncomplicated implant procedure as long as there is no pneumothorax.12 Restrictions of ipsilateral arm movement and avoidance of heavy lifting precludes use of overhead lockers if travelling alone.

Figure 3. Sample device identification card
Exercise
Patients with cardiac rhythm management devices are generally safe to exercise. In the initial post-implantation period, activities with vigorous arm movements ipsilateral to the device should be avoided. All current pacemakers have the capability of varying pacing rates in response to exercise. For patients with pacemakers:
- patients who are more active may need their rate response and other parameters fine-tuned to enable them to achieve maximum benefit from their device
- high-level athletes need to be cleared for participation
- pacemaker-dependent athletes should avoid sports in which there is a risk of collision and possible direct damage to the pacemaker.13
For patients with ICDs:
- patients are advised to keep their heart rates at least 15 beats below (usually 165 bpm) the rate the device is programmed to provide an intervention (usually 180 bpm or higher)
- contact sports should be avoided
- current international guidelines recommend moderate physical activity only, thus precluding most competitive sports (exceptions include cricket and golf).13
Sexual intimacy
People with pacemakers can continue their regular sexual activity. Heart rate increases with sex; hence, ICD intervention rates should be programmed to a level high enough to prevent shock during sex. If the patient does receive a shock, the patient’s partner may feel a tingling sensation.
Psychosocial impact
Up to one-third of ICD recipients experience depression, anxiety and post-traumatic stress disorder.14
Electromagnetic interference
Household and hobby appliances rarely affect modern pacemakers and defibrillators if they are in good working condition. Generally, even strong electromagnetic interference (EMI) will only affect devices within a short distance and while devices are in that range. Electrical interference is a problem when devices sense external signals as intrinsic cardiac signals, resulting in inhibition of pacing and possibly inappropriate defibrillation. Magnets flick a switch in pacemakers and defibrillators, which can result in asynchronous pacing or inactivation of defibrillation. More comprehensive EMI information is available online.15
Recommendations for common EMI situations and appliances (Table 2):
- Mobile phones – keep at least 15 cm away from device (use on opposite ear, do not carry in ipsilateral shirt pocket).
- Anti-theft devices (eg in department stores) – pass through at a normal pace but do not linger.
- Airport security – causes no harm to devices if passing through without lingering, may set off alarm because of the metal in devices. Inform airport staff and show device ID card. Usually, security staff will perform a hand search or use a hand-held wand. Advise patients to place their hand vertically over device to avoid the wand passing too close to the device. X-ray security scanners are safe for device patients.
- Medical and dental procedures (eg surgery using diathermy, medical radiation treatment, magnetic resonance imaging [MRI] scanning, transcutaneous electrical nerve stimulation, electroconvulsive therapy) often require advice from the cardiologist as programming changes may be required and certain restrictions may apply.
MRI scans
There are approximately 200,000 pacemakers and defibrillators implanted in Australians, and for over half of these people, there will at some point be cause for an MRI scan.16 Historically, this scan has not been possible. The first MRI-conditional pacemaker system was approved in Australia by the Therapeutic Goods Administration (TGA) in 2011. Patients must have an entire system (leads and pulse generator) with no redundant leads as well as fulfil certain conditions to be eligible for an MRI scan. Thus, cardiology and radiology departments have developed safe screening protocols, with some departments yet to allow scanning of cardiac device patients even if they have an MRI-conditional system. Most newly implanted pacemaker systems are MRI conditional; however, in most cases, a pacemaker generator replacement unfortunately does not afford MRI conditionality. ICD systems have only more recently achieved TGA approval, with fewer than half of the current new implants having local TGA approval, although many have been approved in Europe and the United States.17
Palliation and ICDs
In patients approaching end of life, it may not be appropriate for ICDs to remain active and deliver shocks to the heart. Inactivating an ICD may allow patients to have a peaceful death. About 20% of ICD patients receive shocks in the last weeks, days or hours of their lives. The unwanted resuscitation of a terminally ill patient can be distressing for both the dying and their families.18 This decision can be made by the patient’s treating physician in consultation with the patient, their family and the patient’s cardiologist. If the patient is not in hospital, usually the cardiac device cardiologist will need to approve inactivation of the ICD. Although most physicians are comfortable inactivating an ICD, few if any would consider turning off pacing. The New South Wales government has specific guidelines detailing the deactivation process.19
Cardiac devices need to be removed prior to cremation due to potential damage to the crematory chamber from flying metal. If there are no plans for cremation, a patient may be buried with their device in situ.
The future of cardiac device management
New technologies are being developed that will change the face of pacing altogether. The ‘weak link’ of current cardiac devices is the transvenous lead. Defibrillator leads are the most susceptible, with 10-year failure rates up of to 20%.20 These failures relate to mechanical stress causing lead failure, infection including endocarditis, and complications relating to lead insertion or removal procedures, including pneumothorax, perforation, valve disruption and thrombus formation.
Future cardiac device systems will be constructed on a ‘modular’ basis. Only what is required at each point in time will be implanted, with addition of components as needed (Figure 4). For example, a subcutaneous defibrillator would be implanted in a post–cardiac arrest patient. If ventricular tachycardia then becomes evident, a leadless right ventricular pacemaker could be added that communicates wirelessly with the defibrillator and is able to deliver antitachycardia pacing as well as ventricular bradycardic support. Should there be subsequent requirement for atrial bradycardic support or cardiac resynchronisation, an atrial leadless pacemaker and/or a left ventricular catheter-delivered pacemaker can also be added.
The future of cardiac rhythm management devices is exciting indeed.
Figure 4. Future cardiac device systems will be constructed on a ‘modular’ basis using leadless components
Reproduced with permission from Wolters Kluwers Health, from Tjong FVY, Reddy VY. Permanent leadless cardiac pacemaker therapy: A comprehensive review. Circulation 2017;135:1458–70