In 2014, 46% of Australians received at least one antibiotic prescription.1 This prescribing rate is higher per capita than in many comparable countries, including England, Canada, Sweden and the Netherlands.1 The volume prescribed is higher than the Organisation for Economic Co-operation and Development average.2 The difference is considerable, with consumption in Australia apparently twice that of Canada and three times that of Sweden.1 There are no obvious reasons why antibiotic use should be higher in the Australian community setting; therefore, it is likely that some antibiotic use may be unnecessary.
Antibiotic use exposes patients to the risk of adverse effects and the development of resistance. This may include drug side effects and the development of antibiotic resistance among bacteria in that individual and in the population more broadly.3 Antimicrobial stewardship (AMS) aims to guide the use of antimicrobial drugs to optimise patient outcomes while minimising any adverse effects. Most human antimicrobial use is from antibiotics prescribed in primary care; therefore, general practitioners (GPs) have a critical role in AMS.4
To address AMS, we need accurate data on antibiotic prescribing by Australian GPs, but this is not available from current data sources. Three main sources of data are the Pharmaceutical Benefits Scheme (PBS)/Repatriation Pharmaceutical Benefits Scheme, the MedicineInsight program and the Bettering the Evaluation and Care of Health (BEACH) survey, but each has significant limitations. PBS data are administrative data with no clinical information. Up to 25% of PBS prescriptions are from non-GP providers, and private prescriptions (outside the PBS restrictions) are not included.5 The MedicineInsight program,6 managed by NPS MedicineWise, aggregates data from GP electronic medical records (EMR) for reports as required.1 While Australia-wide, it is a voluntary program with antibiotic prescriptions only one part of its remit. BEACH was a paper-based survey of 100 consecutive patient presentations from a representative sample of 1000 GPs per year. It collected data including problems managed and medications prescribed, but was discontinued in 2016. While the extensive data are still available, the survey cannot be used for ongoing monitoring.7
A range of clinical software is used in Australian general practices. As a result of limited interoperability, this has limited the secondary analysis of EMR.8 There is a need to develop a sustainable, ongoing way to accurately monitor GP prescribing and to meaningfully interpret the data available.
The aim of this research was to extract routinely collected data from general practice EMR and to use this to describe antibiotic prescribing patterns.
Method
We retrospectively analysed routinely collected GP data from the EMR of patients from 50 general practices across Melbourne’s inner eastern suburbs using POLAR (Population Level Analysis and Reporting for general practice formerly known as MAGNET).9
Patient-level data were extracted for consultations and antibiotic prescriptions between 1 January 2010 and 31 December 2014. Unique linkage keys linked consultation data with antibiotic prescriptions and could track patients across practices. Consultation data included dates of consultations and the age of the patient. Antibiotics were identified and coded according to the Anatomical Therapeutic Chemical Classification.10 Only the original prescription was included; repeats on the same prescription were not examined as the majority of repeat antibiotic prescriptions were not dispensed.5 Systemic and topical antibiotic prescriptions were included in the analysis, except for topical chloramphenicol, which is available without prescription in Australia. During this pilot research, some links between the consultation data and the antibiotic data were inadvertently broken. Where the antibiotic prescription did not link directly to a consultation, the previous or subsequent consultation in that year was used to determine the patient’s age at time of consultation. If there was no other consultation in that year by the patient, or no age was available, the age was recorded as missing. Ages were examined in four broad ranges: under 1, 1–19, 20–49 and ≥50.
Descriptive analysis of the data was undertaken using Stata 13.1 (StataCorp). We examined the numbers of each antibiotic prescribed and the number of consultations. Antibiotic prescribing was assessed across the days of the week, months and years and by the age of the patient.
The Monash University Human Research Ethics Committee granted ethics approval for this research (number CF12/1057 – 2012000504).
Results
Antibiotic prescribing data were complete for 44 of the 50 practices, with 615,362 antibiotic prescriptions provided to 166,772 patients over the five years. Ten antibiotics accounted for 518,016 (84.2%) of the antibiotic prescriptions (Table 1). Cefalexin (146,155, 23.8%), was the most frequently prescribed antibiotic. The number of cefalexin prescriptions remained constant over the five-year period (Figure 1). Trimethoprim and metronidazole, the sixth and tenth most commonly prescribed antibiotics respectively (Table 1), also remained constant over time (Figure 2). By contrast, the remaining seven most commonly prescribed antibiotics – amoxicillin–clavulanic acid, roxithromycin, doxycycline, clarithromycin (Figure 1), cefaclor, erythromycin, and phenoxymethylpenicillin (not shown) – had prescribing peaks in winter. Amoxicillin–clavulanic acid (93,380, 15.2%) was more commonly prescribed than amoxicillin (7390, 1.2%) and other forms of penicillin (Table 1). Phenoxymethylpenicillin (22,090, 3.6%) was the only narrow-spectrum beta-lactam antibiotic in the ten most frequently prescribed antibiotics (Table 1). The number of prescriptions and winter peaks for roxithromycin (Figure 1), cefaclor and erythromycin declined over time, but they were still being prescribed in large numbers in 2014. The most commonly prescribed quinolone was norfloxacin, with 9843 prescriptions over five years (1.6% of total; Table 1). Macrolides (roxithromycin, clarithromycin and erythromycin) comprised 129,132 (21%) prescriptions (Table 1).
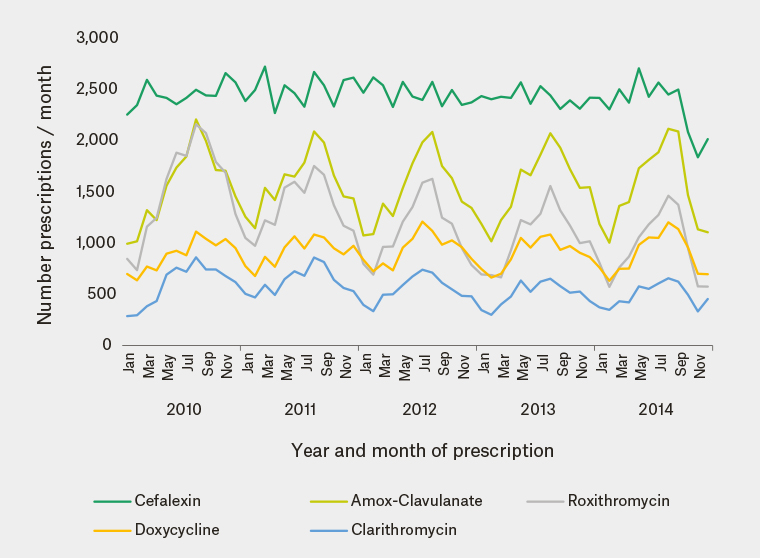
Figure 1. The five most frequently prescribed antibiotics by year and month of prescription (44 practices)
Note: Summer is December to February and winter is June to August.
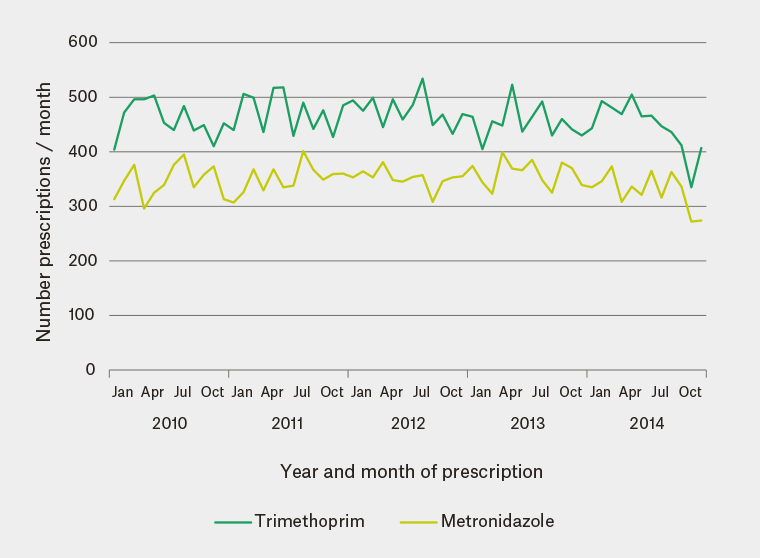
Figure 2. Trimethoprim and metronidazole prescriptions by year and month of prescription (6th and 10th most frequently prescribed)
|
Table 1. Antibiotics by frequency of prescription 2010–14 (44 practices,
n = 615,362)
|
|
Antibiotic
|
Frequency
|
Percentage
|
|
Cefalexin
|
146,155
|
23.8
|
|
Amoxicillin-Clavulanic acid
|
93,380
|
15.2
|
|
Roxithromycin
|
72,089
|
11.7
|
|
Doxycycline
|
54,389
|
8.8
|
|
Clarithromycin
|
33,215
|
5.4
|
|
Trimethoprim
|
27,679
|
4.5
|
|
Cefaclor
|
24,354
|
4.0
|
|
Erythromycin
|
23,828
|
3.9
|
|
Phenoxymethylpenicillin
|
22,090
|
3.6
|
|
Metronidazole
|
20,837
|
3.4
|
|
Mupirocin
|
18,899
|
3.0
|
|
Framycetin
|
14,062
|
2.3
|
|
Flucloxacillin
|
12,719
|
2.1
|
|
Norfloxacin
|
9,843
|
1.6
|
|
Amoxicillin
|
7,390
|
1.2
|
|
Tinidazole
|
6,691
|
1.1
|
|
Minocycline
|
6,163
|
1
|
|
Trimethoprim/sulfamethoxazole
|
5,589
|
0.9
|
|
Other antibiotics
|
15,990
|
2.6
|
Consultation data were complete for 39 of the above 44 practices, with 6,227,104 recorded consultations in the five-year period involving 472,197 patients. A total of 164,522 (34.8%) of the patients received 597,302 antibiotic prescriptions.
The antibiotic prescribing rate per consultation varied with age, which was available for 590,105 (98.8%) of the prescriptions. Patients aged 1–19 years had fewer consultations but received antibiotics at a higher rate per consultation than infants aged <1 and adults aged >19 years. The rate of antibiotic prescribing per consultation per year fell between 2010 and 2014 in age groups ≤49 years, with the largest declines occurring in age group 1–19 years. Age group ≥50 years showed little variation in the antibiotic prescribing rate per consultation over time. Cefalexin was the most prescribed antibiotic across all age groups.
Examination of the reason-for-prescription field (which did not include the progress notes) revealed that for 494,085 (82.7%) of the prescriptions, no reason was available.
Discussion
This research shows the utility of data extracted from general practice EMRs and that a descriptive analysis, using this cohort as the example, can provide information to direct AMS activities.
Not only is the Australian prescribing rate high in comparison to European countries,1,2 with most antibiotics prescribed by GPs,11 but there is a preference for broad-spectrum antibiotic agents in this cohort. In 2013, on the basis of PBS data, amoxicillin, cefalexin and amoxicillin-clavulanic acid were the most dispensed antibiotics Australia-wide.5 However, amoxicillin accounted for only 1.2% of the total antibiotic use in this cohort. Explanations for this cannot be obtained from the available data. Why this cohort of practices showed such low rates of penicillin, amoxicillin and flucloxacillin prescribing and high rates of cefalexin and amoxicillin-clavulanic acid prescribing needs further investigation to understand the clinical and non-clinical drivers. Macrolides were commonly prescribed. This local preference for broad-spectrum antibiotics contrasts with self-reported intentions of Australian GPs, with 70% reporting in 2013 that they would always/often prescribe narrow-spectrum antibiotics.12 It is possible that GPs may not perceive cephalosporins, amoxicillin-clavulanic acid and macrolides to be broad-spectrum agents. In 2012 in Europe, narrow-spectrum penicillins were the most frequently used group of antibiotics in the community.13,14 Infections encountered in community practice in Europe would be expected to be similar to those in Australia, and therefore it would be expected to be safe to use these for many common conditions (informed by any local differences in pathogen resistance patterns). Promotion of narrow-spectrum penicillins could be a local AMS target. The prescription of quinolones is restricted in Australia, and no quinolone was represented in the ten most frequently prescribed antibiotics.
The lack of seasonality in prescribing of cefalexin, trimethoprim and metronidazole probably reflects use in year-round infections such as skin, urinary tract, genital and intestinal infections. Of note, the Therapeutic Guidelines recommended flucloxacillin as the first-line antibiotic for skin and soft tissue infection;15 however, it was not among the most commonly prescribed antibiotics in these practices.
The winter prescribing peaks suggest prescribing for respiratory tract infections, which has been described in Australian16 and international studies.17,18 During 2010, Therapeutic Guidelines ceased recommending the use of cefaclor and roxithromycin for pneumonia, but in 2014 they were still being prescribed with a winter peak frequency. It suggests that this cohort of prescribers had either incomplete awareness of the changed guideline or used different guidelines. Therapeutic Guidelines did not recommend amoxicillin–clavulanic acid for community respiratory tract infections,15 but the winter peaks in prescribing suggest that it was being prescribed for this reason. A study of which guidelines these GPs use and how guideline changes are notified to them seems indicated.
The 2014 PBS figure of 46% of the Australian population being dispensed at least one antimicrobial agent per year1 is higher than the 34.8% of patients in this cohort.19 This may be due to PBS including dispensing data from other providers (such as community-based specialists, emergency department and private hospital inpatients).5 MedicineInsight data (a larger Australia-wide general practice electronic medical record extraction program) showed that 30% of patients were prescribed systematic antimicrobials in 2014.1 This is comparable to our data, which included topical antibiotics.
The reason for the decline in prescribing rate between 2010 and 2014 in age groups <20 years is unknown. It may relate to a decline in general practice presentations for upper respiratory tract infections, throat complaints and ear pain/earache between 2006–07 and 2015–167 or the fall in cefaclor and roxithromycin prescriptions seen in this study. Community campaigns during this period were discouraging antibiotics for common colds, which may have influenced behaviour. This would require a more detailed investigation.
This research examined routinely collected data from the EMR of general practice patients. In Australia, patients may attend any general practice; an advantage of POLAR is that it could track patients across practices within the POLAR catchment. A major limitation is that the data extracted are entirely dependent on the clinical software package and what GPs chose to document and where.20 The reason-for-prescription field in some software packages was free text and in all packages was optional, resulting in a low completion rate. We were unable to determine the reasons for the antibiotic prescriptions and note that antibiotics may be prescribed for common conditions such as acne rosacea or acne vulgaris and for prophylaxis (eg malaria). This limitation requires information technology and standardisation solutions beyond the scope of this research. However, despite this serious limitation, we have shown how data available in general practice EMR might be used for AMS. Of the 50 practices in the dataset, six did not have a complete medication dataset, and a further five practices had incomplete consultation data. This may be due to software changes/updates between entry and extraction,8,21 and requires further investigation. This dataset represents the doctors and patients in a defined urban area, so may not be typical of other regions. However, this may facilitate targeting of AMS initiatives to specific practices or defined localities. Comorbidities were not examined in this study but would be a valuable addition to future studies. The data presented here are antibiotic prescriptions written by GPs. There is no linkage with dispensed data, so it is not known how many of these prescriptions may have been delayed prescriptions provided to a patient with instructions of only filling the prescription if the patient’s condition deteriorated, or if a patient chose not to fill the prescription.
Implications for general practice
This study shows that Australian general practice EMR data can be extracted, and that a descriptive analysis of antibiotic prescriptions can identify targets for intervention in AMS programs and monitor change over time. For example, in this cohort, we revealed high use of broad-spectrum antibiotics and winter prescribing peaks. The continued prescribing of cefaclor and roxithromycin in winter peaks suggests incomplete awareness of changes in Therapeutic Guidelines for treatment of respiratory infections, or the use of alternative guidelines. Information for AMS would be significantly enhanced if reasons for prescription were documented in the EMR in a standardised field. GPs should be encouraged to complete the reason-for-prescription field. Software changes are required to improve data capture at the GP-software interface. GPs and Primary Health Networks should be encouraged to conduct AMS audits of antibiotic prescriptions from EMR.