Chronic hepatitis C (CHC) was previously a condition primarily managed by specialists. However, the listing of direct-acting antiviral (DAA) regimens on the Pharmaceutical Benefits Scheme (PBS), expanded prescriber eligibility and the release of Australian recommendations for the management of hepatitis C virus (HCV) infection in 20161 have expanded the role of general practitioners (GPs) in the management of patients with CHC. The shift to primary care of uncomplicated non-cirrhotic CHC aims to improve overall treatment access and uptake.2
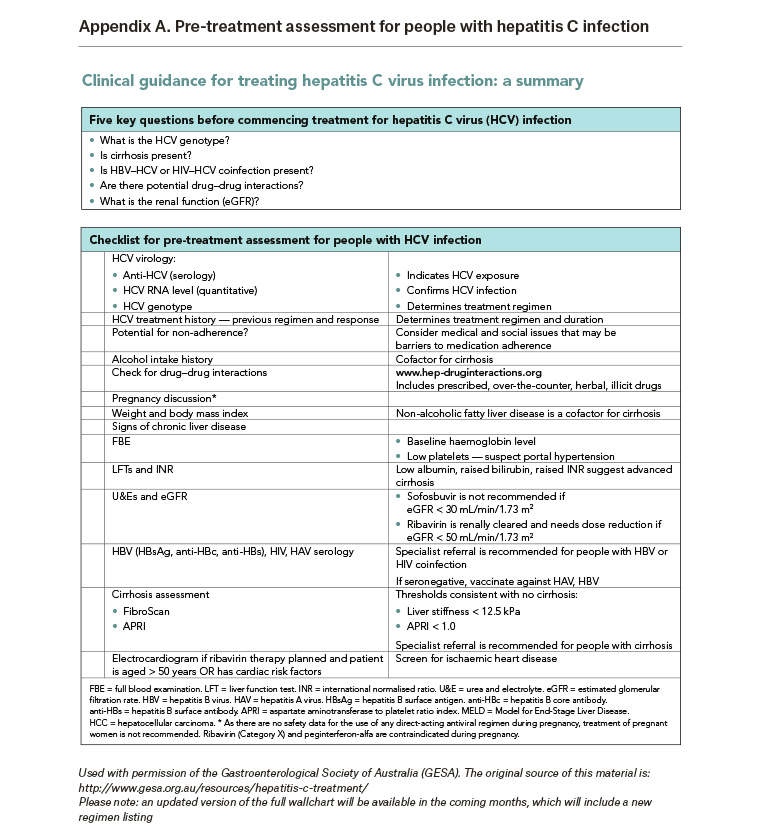
The latest estimates indicate there were 199,412 people living with CHC in Australia at the end of 2016.2 Between March 2016 and June 2017, 43,360 patients started DAA treatment.3 However, treatment initiation rates have more than halved, from 3000–5000 patients per month in the first six months following PBS listing to 1500–2000 patients per month as at June 2017.3 Patients still living with CHC are likely to be at varying stages in the diagnosis and care continuum, and may require further assessment in order to be ready for treatment. Appendix A outlines pretreatment assessment for patients with HCV infection.
The aim of this study was to assess where patients with CHC are situated in the diagnosis and care continuum, highlight opportunities to improve pretreatment assessment and thereby increase overall DAA treatment uptake.
Method
A cross-sectional observational study was conducted using MedicineInsight, a national general practice data program developed and managed by NPS MedicineWise, with funding support from the Australian Government Department of Health. MedicineInsight is the first large-scale national general practice data program in Australia to extract and collate longitudinal, de-identified patient health records from clinical information systems (Best Practice, Medical Director 3 and Genie).4
The MedicineInsight program collects de-identified data on patient demographics, encounters (not including progress notes), diagnoses, prescriptions, pathology tests and referrals, and includes records for about 3.6 million regular patients (approximately 15% of the Australian population) from more than 3300 GPs in 653 general practices across Australia.
This study was approved by Bellberry Human Research Ethics Committee (application number: 2016-11-792, approved 27 April 2017).
Participants
Patients with a diagnosis of HCV infection in their medical record were identified from general practices enrolled in the MedicineInsight program. For the purpose of this study, we included patients aged 18 years or older who had attended their general practice at least three times between 1 January 2013 and 31 August 2017, and who had a new hepatitis C-related entry recorded in this period. Appendix B and Table B1 (available online only) outline how patients with HCV infection were identified using MedicineInsight.
Patients were classified into two groups on the basis of the available information for their infection status: either ‘indeterminate’ (meaning their precise hepatitis status could not be determined from the information available) or ‘confirmed CHC’. Additional criteria were applied to patients initially classified as ‘indeterminate’ to further improve allocation of patients (Figure 1). Patients whose records showed they no longer had HCV, either through treatment or spontaneous viral clearance, were excluded from the study.
Study outcomes
The main outcome is the number of patients who had undergone pre-treatment assessment and management as recommended by current Australian guidelines1 (Appendix A).
Statistics
Descriptive statistics were used to present the study outcomes, including use of percentages and associated 95% confidence intervals (CI), and means and standard deviations (SDs). Robust standard errors were used in the calculation of 95% CIs to adjust for clustering by practice. Data management and analyses were conducted with SAS Enterprise Guide 7.1 (Cary, NC USA, 2015).
Results
Of the 2.63 million patients in MedicineInsight practices who were aged ≥18 years with at least three practice visits between 1 January 2013 and 31 August 2017, 4025 patients were classified as having confirmed CHC during the study period, while 3137 patients were classified as having indeterminate hepatitis C infection (Figure 1).
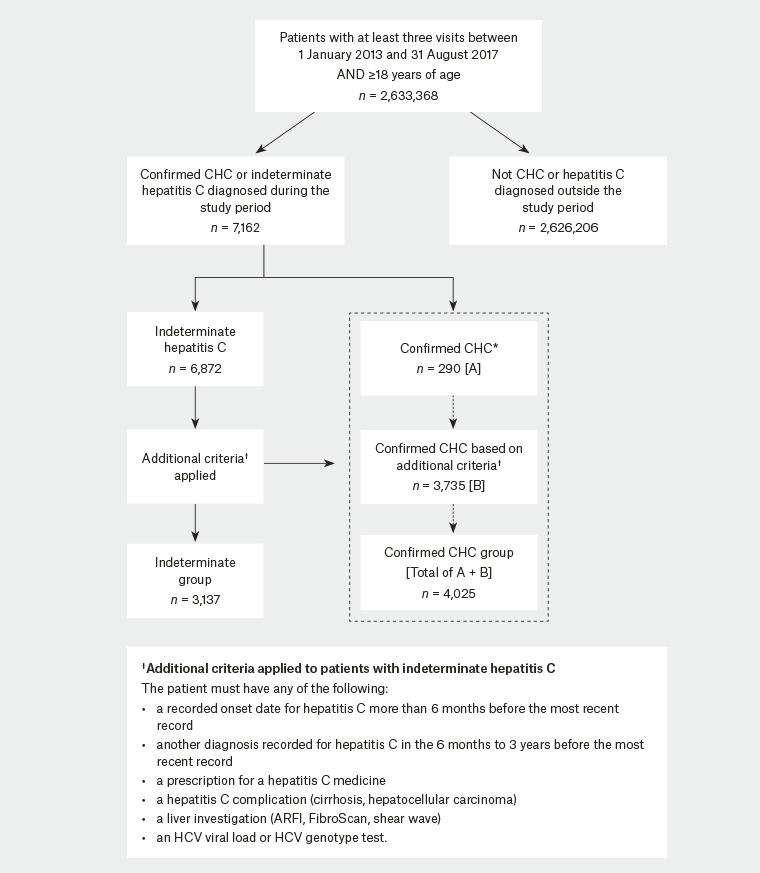
Figure 1. Flow chart of study participants with chronic hepatitis C (CHC), showing method of classification into ‘indeterminate’ or ‘confirmed’ CHC categories.
*Based on codes presented in Appendix B – Table B1
Patient characteristics
Approximately two-thirds of adult patients diagnosed with confirmed CHC during the study period were male (64.2%, 95% CI: 61.9, 66.5), with a mean age of 49 years (SD 12), and more than half were residents of a major city (59.0%, 95% CI: 51.0, 66.9; Table 1). Furthermore, 8.7% (95% CI: 6.6, 10.7) of patients with confirmed CHC identified as Aboriginal and/or Torres Strait Islander.
Co-infection with human immunodeficiency virus (HIV) or hepatitis B virus (HBV) infection was recorded for 2.7% (95% CI: 0.6, 4.8) and 2.5% (95% CI: 1.9, 3.1) of patients, respectively. Additionally, 9.2% (95% CI: 8.1, 10.4) of patients had a record of liver cirrhosis and 1.5% (95% CI: 1.1, 1.9) had a record of hepatocellular cancer.
|
Table 1. Characteristics of eligible patients with confirmed chronic hepatitis C in the MedicineInsight dataset (n = 4025)
|
|
Characteristic
|
|
|
|
Age (years)
|
|
|
|
Age, mean (standard deviation)
|
49 (12)
|
|
|
Age, median (Q1–Q3)
|
50 (40–58)
|
|
|
Gender
|
n (%)
|
95% confidence intervals
|
|
Male
|
2583 (64.2)
|
61.9, 66.5
|
|
Female
|
1434 (35.6)
|
33.3, 38.0
|
|
Missing/indeterminate
|
8 (0.2)
|
0.0, 0.4
|
|
Region
|
|
|
|
Major city
|
2373 (59.0)
|
51.0, 66.9
|
|
Inner regional
|
1083 (26.9)
|
20.5, 33.3
|
|
Outer regional
|
483 (12.0)
|
7.5, 16.5
|
|
Remote
|
69 (1.7)
|
0.5, 3.0
|
|
Very remote
|
17 (0.4)
|
0.02, 0.8
|
|
Indigenous status
|
|
|
|
Aboriginal and/or Torres Strait Islander
|
348 (8.7)
|
6.6, 10.7
|
|
Not Aboriginal or Torres Strait Islander/not known
|
3677 (91.4)
|
89.3, 93.4
|
|
Comorbidity
|
|
|
|
Human immunodeficiency virus infection
|
109 (2.7)
|
0.6, 4.8
|
|
Hepatitis B infection (ever)
|
101 (2.5)
|
1.9, 3.1
|
|
Hepatitis C–related conditions
|
|
|
|
Cirrhosis
|
371 (9.2)
|
8.1, 10.4
|
|
Hepatocellular cancer
|
60 (1.5)
|
1.1, 1.9
|
Diagnosis of suspected chronic hepatitis C
Following a positive HCV antibody test, an HCV polymerase chain reaction (PCR) test is essential to confirm current hepatitis C infection.1 In this MedicineInsight cohort, 22.6% (708/3137, 95% CI: 20.2, 25.70) of those who were assigned to the indeterminate group and 49.9% (2007/4025, 95% CI: 46.5, 53.2) in the confirmed CHC group had an HCV qualitative PCR recorded (Figure 2A).
Pretreatment assessment for confirmed chronic hepatitis C
Virological evaluations
Of the 4025 patients in the confirmed CHC group, 65.8% (2649 patients, 95% CI: 63.0, 68.7) had ever had an HCV quantitative PCR (viral load) test recorded, 40.3% (1623 patients, 95% CI: 35.5, 45.2) had ever had an HCV genotype test recorded and only 33.3% (1342 patients, 95% CI: 28.3, 38.4) had ever had both HCV viral load and HCV genotype tests recorded in clinical data fields collected by MedicineInsight (Figure 2B).
Other investigations
Records of monitoring tests were explored over the six months after diagnosis to reflect baseline/pretreatment assessments. Of all the recommended baseline monitoring tests, liver function tests (LFTs) were ordered most frequently in the six months after the diagnosis of CHC (53.6%, or 2159 patients, 95% CI: 51.0, 56.3); followed by full blood count (FBC) (51.8%, or 2085 patients, 95% CI: 49.3, 54.3); and urea, electrolytes and creatinine (UEC) (47.4%, or 1908 patients, 95% CI: 44.7, 50.1; Figure 2C). By comparison, tests to assess co-infection with HIV or hepatitis A or B virus were performed less frequently (13.0%, or 524 patients; 0.5%, or 21 patients; and 12.7%, or 509 patients, respectively), although a further 12.2% of patients (489 patients) had unspecified hepatitis serology tests (Figure 2C). Only 10 patients (0.3%, 95% CI: 0.0, 0.5) had all recommended baseline tests (HCV viral load, HCV genotype plus all the tests in Figure 2B and 2C) recorded during this time.
Liver fibrosis assessment
Overall, among the confirmed CHC group, our assessment showed that 56% (95% CI: 52.5, 59.3) had a calculated aspartate aminotransferase (AST) to platelet ratio index (APRI) score ≤1, indicating low probability of cirrhosis (Figure 2D).1 Excluding patients in whom an APRI score was not assessable (eg AST and/or platelet test result were unavailable in the past two years), 84.6% of patients had an APRI score ≤1.
Only 35 patients (0.9%, 95% CI: 0.0, 1.7) had a record of a FibroScan (Echosens, Paris), shear wave elastography or acoustic radiation force impulse (ARFI) imaging in data fields collected by MedicineInsight (Figure 2E).
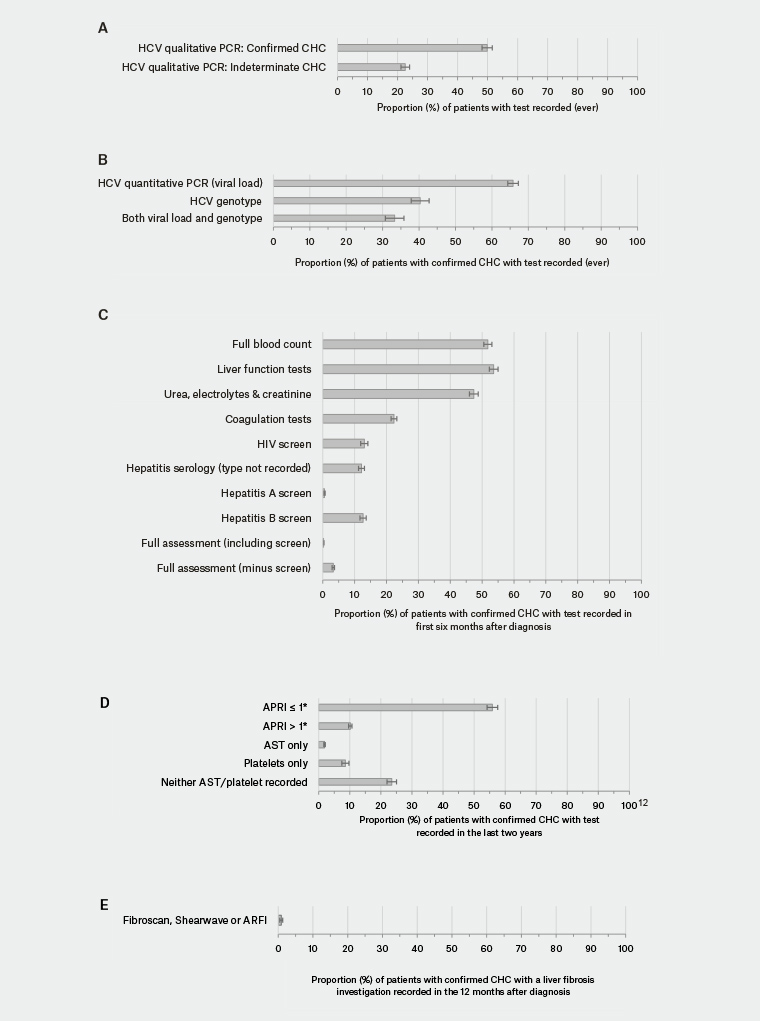
Figure 2. Pre-treatment assessments in patients with indeterminate (A) or confirmed (A–E) chronic hepatitis C (CHC)
* APRI = [(AST level (IU/L) ÷ AST (upper limit of normal, ie 40 IU/L)) ÷ platelet count (109/L)] × 100
ARFI, acoustic radiation force impulse; APRI, aspartate aminotransferase to platelet ratio index; AST, aspartate aminotransferase; CHC, chronic hepatitis C; HCV, hepatitis C virus; PCR, polymerase chain reaction
Treatment and referral data
Treatment
Of the 4025 patients with confirmed CHC, 27.4% (1102 patients, 95% CI: 24.4, 30.3) had a record of any medicine for CHC, and 26.2% (1054 patients, 95% CI: 23.2, 29.2) had treatment with an interferon-free DAA regimen (Figure 3A). For a list of medicines included in this study, see Appendix C (available online only).
Specialist referral
MedineInsight data indicate that 9.6% (385 patients, 95% CI: 7.5, 11.6) of patients with confirmed CHC had a referral to a gastroenterologist, and 1.9% (76 patients, 95% CI: 0.9, 2.8) had a referral to an infectious diseases physician (Figure 3B) recorded in the 12 months after diagnosis. A further 41.9% of patients had a referral recorded, but the specialist type could not be ascertained from data collected by MedicineInsight.
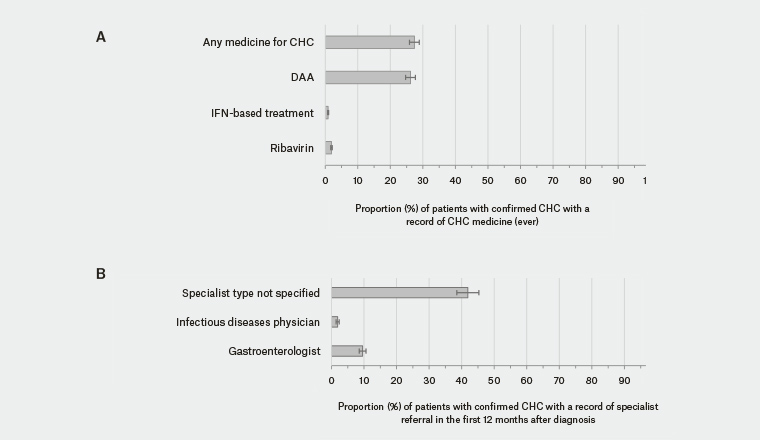
Figure 3. Management of patients with confirmed chronic hepatitis C (CHC). Data shown are records of medicines (A) and referrals (B)
DAA, direct-acting antivirals; IFN, interferon
Discussion
This study sought to use general practice data to assess where patients with CHC are situated in the diagnosis and care continuum, and to highlight opportunities for improved clinical care. Overall, our results indicate that incomplete testing and assessment are evident across the stages of CHC management, which is in part likely to reflect the recent shift in CHC management to primary care. For example, although all patients with suspected CHC (positive HCV antibody) should have a confirmatory HCV PCR test, an HCV qualitative RNA test was found for half (49.9%) and an HCV quantitative RNA for two-thirds (65.8%) of the confirmed CHC cohort. While our results are likely to underestimate actual HCV PCR testing, data published by the Kirby Institute also indicate incomplete RNA testing.2
In the six months after diagnosis, about half of the patients with confirmed CHC had LFTs, FBC and/or UEC tests recorded. Similarly, the proportion of patients with records showing screening for co-infection with HIV or hepatitis A or B virus was surprisingly low (13%, 0.5% and 12.7%, respectively), with a further 12.2% of patients having unspecified hepatitis serology tests recorded. However, it should be noted that these investigations might have been performed outside this period or in other care settings (eg in other practices or hospitals).
Of the patients with confirmed CHC who had AST and platelet results available, 86% had an APRI score ≤1, which indicates the majority of patients have a low probability of cirrhosis and are likely to be suitable for management in primary care. Without easy access to FibroScan, an APRI score of ≤1 can rule out the need for FibroScan when clinical assessment and other investigations suggest a low likelihood of cirrhosis. Data released by the Kirby Institute also suggest that the majority of patients with CHC yet to be treated are those without cirrhosis.3
Over a quarter (26.2%) of patients in the confirmed CHC group had been prescribed an interferon-free DAA regimen. As more than 90% of patients completing DAA therapy are expected to be cured of CHC, most of these patients will be those who are either yet to finish their course of therapy and have a follow-up HCV PCR at 12 weeks post-treatment, or whose recorded HCV status in their general practice record has yet to be updated to reflect treatment response.
Limitations
We acknowledge that a key methodological constraint was our dependence on the data available through the MedicineInsight program. Previous studies found MedicineInsight data viable for estimating the prevalence of musculoskeletal disorders5 and post-market drug surveillance.6 Data availability in MedicineInsight depends on whether diagnoses, tests or assessments have been recorded in the patient’s general practice record and whether they have been recorded in fields from which data can be extracted and analysed. For example, although there is limited access to fibrosis assessment in some settings,7 the level of FibroScan testing found in the study was unexpectedly low; this is likely to reflect the recording of these tests in data fields not collected/extracted by MedicineInsight (eg in progress notes or as PDF attachments) or these assessments having been performed in tertiary care settings. In addition, MedicineInsight does not capture whether patients have declined offered treatment or reasons why a patient may decline testing or treatment when it has been offered. Given the lack of PCR results and resulting reliance on recorded diagnosis and indirect indications of CHC status, some patients may have been inaccurately assigned to one of the two study groups. A follow-up study would be useful to validate the study’s findings with the full medical records of a sample of patients.
Despite these limitations, however, our results strongly indicate that there is a substantial opportunity for GPs to recall more patients with CHC for confirmation of diagnosis and pretreatment assessment. This can improve access to highly effective and well-tolerated DAA regimens for CHC, which are now also available for prescription in the primary care setting.
Implications for general practice
The majority of patients with CHC appear suitable for management of HCV in primary care. Proactive reviews of patient records by GPs to identify patients living with HCV infection are critical to maintain treatment momentum.
Appendix B. Identifying patients with CHC
Coding definitions used to identify most recent hepatitis C status
Patients were identified using the most recent hepatitis C-related coded or free-text entry in the diagnosis (medical history), reason for encounter or reason for prescription fields of the general practice clinical information system.
Although there were no coded (Pyefinch or Docle) terms specifically for ‘chronic hepatitis C’ available for use by GPs in clinical information systems, available coded terms included ‘hepatitis C infection’, ‘hepatitis C’ and ‘hepatitis C carrier’. These were the terms that were most commonly recorded for patients in this study.
In addition, there were also more than 4000 unique free-text entries for hepatitis C-related information recorded in the diagnosis fields (e.g. ‘hepatitis C cured’, ‘hepatitis C and lethargy’, ‘hepatitis C serology’, etc). These free-text terms were analysed manually by a clinical coder based on pre-defined clinical concepts, and were used to assign patients to either indeterminate or confirmed CHC groups (see Table B1: Coding definitions). A quality assurance check was performed by another member of the clinical team to ensure that this approach was consistent.
To assist with this process, a priority list was developed and agreed by internal Medical Advisors at NPS MedicineWise to resolve conflicting diagnosis records used on the same day (available upon request).
|
Table B1. Coding definitions
|
|
|
Groups
|
Clinical concept
|
Sample free-text terms manually coded into study cohorts*
|
|
Included
|
Confirmed chronic hepatitis C (CHC)
|
Includes patients whose most recent recorded diagnosis clearly indicates chronic hepatitis C (e.g. complications, treatments or genotype testing) or who met additional criteria**
|
• Chronic hep c infection
• Hepatitis C cirrhosis
• Hepatitis C vasculitis
• Portal hypertension due to hep C
• Hep C genotype pending
• Hep C Genotype 1
• Hepatitis C – referral for treatment
• Hepatitis C treatment
• Hepatitis C treatment failed
|
|
|
Indeterminate**
|
Includes patients whose most recent recorded diagnosis indicated active or antibody positive hepatitis C but not specifically CHC
|
• Hepatitis C
• Hepatitis C infection
• Hepatitis C carrier
• Hepatitis C positive
• Hepatitis C antibody positive
• Hepatitis C referral
• Hepatitis C monitoring
• Hep C asymptomatic
• Hep C PCR ordered
• Hep C viral load
• Hepatitis C & lethargy
|
|
* The designated diagnosis fields in the general practice clinical information systems include ‘diagnosis’ (medical history), ‘reason for encounter’ or ‘reason for prescription’
** Additional criteria
Additional criteria were applied to confirm chronic hepatitis C in a patient whose most recent recorded diagnosis was indeterminate. The patient must have any of the following:
• a recorded onset date for hepatitis C more than 6 months prior to the most recent record
• another diagnosis recorded for hepatitis C in the 6 months to 3 years prior to the most recent record
• a prescription for a hepatitis C medication
• a hepatitis C complication (cirrhosis, hepatocellular carcinoma)
• a liver investigation (ARFI, FibroScan, Shear wave)
• an HCV viral load or HCV genotype test.
|
|
Appendix C. Medications for hepatitis C and their classification in this study
|
|
Active ingredient
|
Brand name
|
Classification in current study
|
|
Boceprevir
|
Victrelis
|
IFN-based treatment
|
|
Daclatasvir
|
Daklinza
|
DAA
|
|
Grazoprevir with elbasvir
|
Zepatier
|
DAA
|
|
Ledipasvir with sofosbuvir
|
Harvoni
|
DAA
|
|
Paritaprevir with ritonavir, ombitasvir and dasabuvir
|
Viekira Pak
|
DAA
|
|
Paritaprevir with ritonavir, ombitasvir, dasabuvir, ribavirin
|
Viekira Pak-RBV
|
DAA
|
|
Peginterferon alfa-2a
|
Pegasys
|
IFN-based treatment
|
|
Peginterferon alfa-2b
|
PEG-Intron
|
IFN-based treatment
|
|
Ribavirin (tablets only not the inhalation)
|
Ibavyr
|
IFN-based treatment
|
|
Ribavirin with peginterferon alfa-2a
|
Pegasys RBV
|
IFN-based treatment
|
|
Ribavirin with peginterferon alfa-2b
|
Pegatron
|
IFN-based treatment
|
|
Simeprevir
|
Olysio
|
IFN-based treatment
|
|
Sofosbuvir
|
Sovaldi
|
DAA
|
|
Sofosbuvir and velpatasvir
|
Epclusa
|
DAA
|
|
Telaprevir
|
Incivo
|
IFN-based treatment
|