Visual distortion is a common presentation in general practice. Patients can find it challenging to convey the subjective nature of their visual distortions and often describe the changes as ‘blurred vision’ or ‘vision loss’. Metamorphopsia, micropsia, macropsia, scotomas and paracentral scotomas are all symptoms of visual function disturbance described in various macular disorders.1 These visual symptoms can often herald or precede an underlying maculopathy. Visual distortion secondary to macular haemorrhage can lead to potentially irreversible loss of vision if not treated promptly. Visual distortion secondary to macular oedema in central retinal vein occlusion (CRVO) could indicate malignant hypertension, leading to stroke and other end-organ damage if left undiagnosed. Thus, visual distortions should be clarified and not overlooked. It is through a comprehensive assessment that a general practitioner (GP) can identify red flags to potentially sight-threatening or life-threatening conditions and refer appropriately. This article focuses on visual distortions secondary to maculopathies and other ocular causes; however, it is important to consider neurogenic causes in assessment.
History
Obtaining a detailed history is important to elicit potential causes of visual distortions. A patient’s demographic, including their age, sex and race, should be noted. A systemic history is important to highlight any co-existing risk factors, such as diabetes, hypertension and hypercholesterolaemia. A personal and family ocular history should be obtained. A social history, inclusive of smoking history, is also relevant. A detailed medication history is important as numerous medications are associated with visual phenomena. Vasodilators such as erectile dysfunction drugs (eg sildenafil) may result in cyanopsia, a distinctive bluish tinge in vision. Vasodilators such as beta blockers and anti-anginal medications are known to cause phenomena such as shimmering, halos or scintillations. Systemic corticosteroids are associated with central serous chorioretinopathy (CSCR) and resultant visual distortions.
Nature of visual distortion
Distortions in vision can be described in terms of shape (metamorphopsia), size (micropsia or macropsia) and colour (dyschromatopsia), and possible aetiologies can be localised to the orbit itself or to the occipital cortex. Metamorphopsia is defined as a deviation of either vertical or horizontal lines in central vision as described by the patient, and commonly indicates macular pathology.1 Patients may complain of typically straight objects, such as doorways or window frames, looking bent or twisted, or simply objects ‘changing shape’. Distortions can manifest as objects appearing disproportionately large (macropsia) or small (micropsia). Dyschromatopsia or colour aberrations can also be implicated, but generally indicate optic nerve pathology. Hemeralopia (day blindness) or nyctalopia (night blindness) can indicate retinal dystrophies. It is important to elicit the location of the visual distortion. Is it a sectoral field change suggestive of detachment, optic neuropathy or retinal vascular disease? Or is it a central or paracentral abnormality, seen in maculopathies or glaucomatous disease? Has the patient’s ability to read deteriorated because of sections of the page being missing or are they having issues with face perception due to a darkened central scotoma? Determining whether the visual disturbance is present in one eye (monocular) or both eyes (binocular) can also aid in localising the pathology. A monocular disturbance suggests that the lesion is anterior to the optic chiasm (eg retina, optic nerve, cornea); in contrast, binocular disturbances are more likely to be neurological. Associated visual symptoms such as photopsia, floaters and transient scintillating colours may indicate aetiologies such as tears, detachments and ocular migraines, respectively.
Onset, duration and evolution of symptoms
A transient visual obscuration such as that experienced in embolic phenomena (eg amaurosis fugax) is likely to require more urgent evaluation than a central visual distortion of 12 months’ duration. Onset, duration and evolution of symptoms helps triage the urgency of referral. A static, central distortion of longer duration can be suggestive of maculopathies such as chronic, dry, age-related macular degeneration (AMD), macular hole, epiretinal membrane (ERM) and diabetic macular oedema (DMO). Conversely, a rapidly worsening central distortion can be indicative of wet AMD with choroidal neovascular membrane (CNVM) or CRVO with insipient cystoid macular oedema (CMO). Associated history of previous visual distortions in a young middle-aged male can be strongly suggestive of CSCR. A history of previous sudden visual loss, in particular sectoral field changes, months or years prior to central distortion development can be a sign of retinal vein occlusion with subsequent CMO.1
Associated symptoms
Any associated symptoms should be noted. Weakness or numbness on one side of the body, diplopia, slurred speech, headaches, difficulty swallowing, word-finding problems and imbalance should be evaluated to rule out a neurological cause of visual distortions.
Examination
Vital and other signs
A patient’s vital signs should be taken. Systemic hypertension is particularly important and may manifest as hypertensive retinopathy/neuropathy and retinal vein occlusion (RVO), leading to macular oedema. It may also be pertinent to check blood sugar levels or to conduct cardiovascular and/or neurological examinations depending on the nature of the presentation.
Visual acuity
Visual acuity testing should be performed using a Snellen chart and with appropriate refractive correction (ie the patient should wear their glasses if that is usual), or using a pinhole. This can be performed using a handheld chart or conventional charts positioned at three and six metres’ distance from the patient. If the patient is unable to see any letters, evaluate whether the patient can count fingers, observe hand movements or simply perceive light. Visual distortions are often coupled with impaired visual acuity.
Intraocular pressure
Various forms of tonometry exist to measure intraocular pressure. Rebound tonometry, such as iCare, is the most prevalent portable device and uses a small plastic-tipped probe that bounces gently against the cornea to obtain a reading.
Pupils
Pupils should be examined for size and their direct and consensual responses. The ‘swinging light test’ can be employed to test for a relative afferent pupil defect (RAPD), whereby a dilatory response is elicited because of a reduction in afferent impulses from the optic nerve. An RAPD is generally observed in optic nerve disease.2
Visual fields
Visual fields to confrontation can help define whether the pathology is neurological or retinal. Sitting directly across from the patient, the GP occludes one eye at a time, mirroring the patient. The patient, looking directly ahead, should indicate when a moving target (usually the GP’s finger) is seen being brought in from the periphery in all four quadrants. The patient’s visual field is compared with the GP’s to assess for any abnormalities.
Colour vision
Colour vision testing using Ishihara plates helps differentiate neurogenic from non-neurogenic vision changes. Acquired dyschromatopsia can be seen with lesions of the optic nerve, retina or the visual cortex. Cerebral dyschromatopsia caused by lesions at the occipital–temporal lobe junction is usually accompanied by other focal neurology, including homonymous visual field defects.1
Amsler grid
The Amsler grid was the first functional test proposed to evaluate visual distortions (Figure 1A).1 It has application in general practice to discover the presence and location of defects in the central field of vision and can also be used for home monitoring by patients. Magnetised Amsler grids can be ordered from the Macular Disease Foundation Australia website and are accessible on various device applications. The Amsler grid allows for simple and rapid qualitative evaluation of alterations of visual function in the central 10 degrees around a fixation point. It consists of a black or white card on which vertical and horizontal parallel lines are drawn, subdivided every 5 mm to form a 10 cm square. The Amsler grid can be used with patients using the following instructions:
- wear your reading glasses and hold the grid at reading distance (approximately 28–30 cm)
- cover one eye
- focus on the black dot in the centre of the grid without moving your eye
- comment on whether there are any broken, wavy, distorted or missing areas1
- repeat the process with the other eye.
Abnormalities can include the absence of a central fixation point (central scotoma), inability to perceive outer areas of the grid (arcuate scotoma), an area of the grid that is missing (paracentral scotoma), or distortions in which the lines appear curvy or wavy (metamorphopsia; Figures 1B, 1C), which can be further described as lines ‘bending’ inwards (micropsia) or outwards (macropsia).3
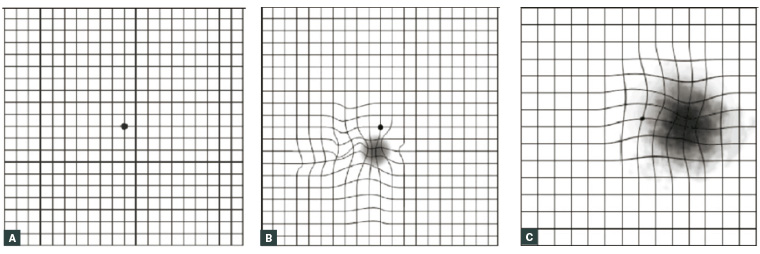
Figure 1. Amsler grid examples
A. Normal Amsler grid; B. Small scotoma below central fixation with surrounding metamorphopsia; C. Large scotoma encroaching central fixation with some metamorphopsia4
Figure reproduced from Broadway DC. Visual field testing for glaucoma – A practical guide. Community Eye Health 2012:25(79–80):66–70. Licence at https://creativecommons.org/licenses/by-nc/3.0/au/
Fundoscopy
Fundoscopy is performed with the lights off or dimmed and the patient in a seated position. Dilate the patient with tropicamide 1% in each eye if pupil examination is normal and acute angle closure and trauma is not suspected. Dilation may take 15 minutes. Instruct the patient to fixate on a precise area, such as the corner of the room. While holding the ophthalmoscope, the GP should examine the patient’s left eye with their own left eye and the patient’s right eye with their own right eye. The presence of a red reflex should be assessed, and the optic disc can be subsequently located by following the blood vessels. The retinal vessels can be assessed and the macula identified by looking temporally to the optic disc. Fundoscopic abnormalities are summarised in Table 1. The PanOptic Ophthalmoscope is particularly useful in general practice as it allows a fundal view that is five times greater than that seen with a standard ophthalmoscope in an undilated eye.
| Table 1. Fundoscopic abnormalities |
| Red reflex |
Reduced red reflex in corneal scarring, cataract, vitreous haemorrhage, asteroid hyalosis |
| Optic disc |
Pallor, swelling (papilloedema), haemorrhage, gross cupping, new disc vessels |
| Vessels |
Arteriovenous nipping, venous congestion and beading, emboli, microaneurysms, haemorrhages, exudate, cotton wool spots, new retinal vessels |
| Macula |
Drusen, exudates, haemorrhage, pigmentary changes |
Differential diagnosis
The most common macular diseases characterised functionally by visual distortions are included in Table 2. Diagnosis can be made using imaging modalities such as optical coherence tomography and various fundus photography techniques.
| Table 2. Common maculopathies causing visual distortions |
| Maculopathy |
Age-related macular degeneration (AMD) |
Macular holes |
Central serous chorioretinopathy |
Retinal vein occlusion (RVO) with cystoid macular oedema |
Diabetic retinopathy with diabetic macular oedema |
Vitreomacular interface disorders |
| Definition |
Acquired disease characterised by neurodegeneration of the photoreceptor–retinal pigment epithelial complex5 |
A retinal break commonly involving the fovea12 |
Characterised by accumulation of subretinal fluid, commonly under the macula15 |
Retinal vein occlusive disease leading to complications of interrupted blood flow including macular swelling17 |
Diabetic disease leading to accumulation of fluid in the macula19 |
Vitreomacular interface abnormalities inclusive of adhesions, traction and membranes21 |
| Types |
- Dry (non-exudative)
- Wet (exudative)
|
- Full-thickness
- Partial-thickness (lamellar holes)
|
|
- Branch retinal vein occlusion
- Central retinal vein occlusion
|
- Non-proliferative diabetic retinopathy
- Proliferative diabetic retinopathy
|
- Vitreomacular traction (VMT)
- Epiretinal membrane (ERM)
|
| Risk factors |
- Increasing age
- Ethnicity
- Genetics
- Smoking
- High intake of saturated and trans fats and omega-6 fatty acids
- Abdominal obesity
- Hyperlipidaemia
- Hyperopia
- Light iris colour
- Cardiovascular disease
- Hormonal status
- Alcohol use
- Vitamin B and D status
- Elevated C-reactive protein6–8
|
- Age
- Female sex
- Myopia
- Trauma
- Ocular inflammation13
|
- Age
- Male sex
- Corticosteroid exposure
- Phosphodiesterase inhibitor use
- Obstructive sleep apnoea
- ‘Type A’ personality traits16
|
- Systemic arterial hypertension
- Atherosclerosis
- Diabetes
- Thrombophilia
- Glaucoma
- Sleep apnoea
- Homocystinuria17
|
- Duration of diabetes
- Proteinuria
- Sex
- Cardiovascular disease
- High glycated haemoglobin levels
- Diuretic use19
|
- Age
- Retinal vascular disease
- Diabetes
- Trauma
- Inflammatory conditions
- Tumours
- Retinal dystrophies21
|
| Symptoms |
- Reduced vision
- Visual distortions
|
- Reduced vision
- Visual distortions
|
- Reduced vision
- Visual distortions
|
- Reduced vision
- Visual distortions
|
- Reduced vision
- Visual distortions
|
- Reduced vision
- Visual distortions
|
| Clinical features |
- Dry AMD: Drusen and geographical atrophy in advanced disease (Figure 2A)
- Wet AMD: Choroidal neovascular membrane and PED (Figure 2B)
|
- Well-defined round or oval lesion in the macula (Figure 2C)
|
- Well-demarcated round or oval-shaped area of neurosensory detachment with or without serous PED (Figure 2D)
|
- Vascular tortuosity
- Retinal haemorrhages
- Cotton wool spots
- Optic nerve oedema
- Macular oedema (Figure 2E)
|
- Macular thickening
- Exudates and cystoid changes
- Microaneurysms
- Haemorrhage
- Hard exudates
- Retinal neovascularisation (Figure 2F)
|
- VMT: Distortion of the fovea, blunted foveal reflex, cystic macular changes, subretinal fluid
- ERM: Reflective sheen over retina, wrinkling of retinal surface (Figure 2G)
|
| Treatment |
- Dry AMD: Preventative strategies; smoking prevention; daily dose of vitamin C, vitamin E, lutein, zeaxanthin, zinc oxide and cupric oxide9
- Wet AMD: Intravitreal anti-VEGF injections, such as ranibizumab, bevacizumab and aflibercept10,11
|
- Surgical repair with vitrectomy with or without internal limiting membrane peel and gas tamponade; post-operative posturing required14
|
- Spontaneous resolution of fluid by three months; potential therapies include focal laser photocoagulation, photodynamic therapy and intravitreal anti-VEGF medications16
|
- Therapies targeted at complications of RVO; intravitreal anti-VEGF therapy, grid laser and intravitreal steroids18
|
- Intravitreal injections, laser photocoagulation and vitreous surgery20
|
- VMT: Paras plana vitrectomy; pharmacologic vitreolysis with ocriplasmin
- ERM: Pars plana vitrectomy with membrane peel21
|
| PED, pigment epithelial detachment; VEGF; vascular endothelial growth factor |
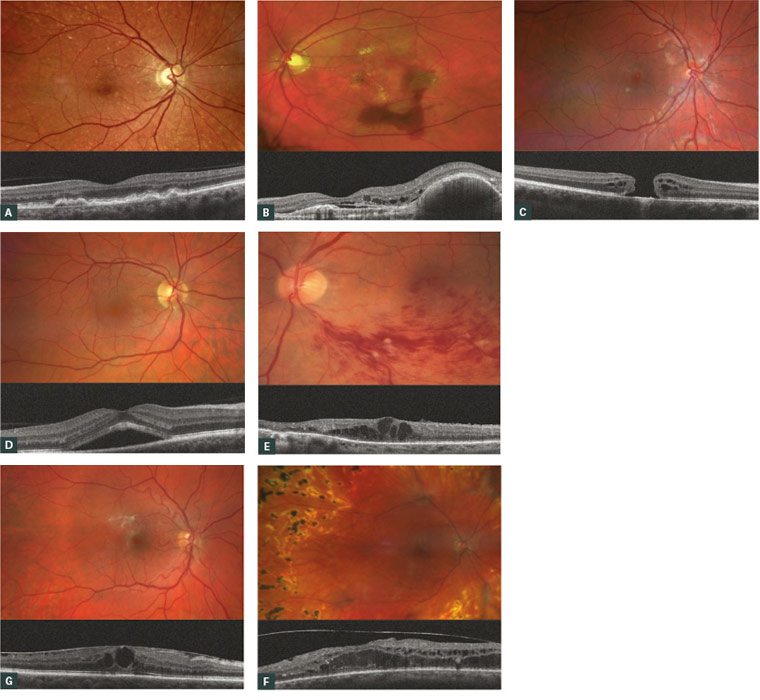
Figure 2. A. Fundus photo and optical coherence tomography (OCT) of drusen in early dry age-related macular degeneration (AMD); B. Fundus photo and OCT of choroidal neovascular membrane in wet AMD with retinal haemorrhage; C. Fundus photo and OCT of full-thickness macular hole; D. Fundus photo and OCT of central serous chorioretinopathy with subretinal fluid; E. Fundus photo and OCT of branch retinal vein occlusion with cystoid macular oedema (CMO); F. Fundus photo of proliferative diabetic retinopathy with new blood vessel formation and panretinal photocoagulation scars; OCT features diabetic macular oedema; G. Fundus photo and OCT of epiretinal membrane with secondary CMO
Referral
Acute visual distortions or sudden deterioration of vision warrant urgent referral for ophthalmological evaluation. These could represent maculopathies such as wet AMD with CNVM or potentially CRVO with CMO, requiring further investigation and management. More indolent, progressive visual distortions may indicate conditions such as macular hole, epiretinal membrane, diabetic macular oedema or worsening dry AMD, requiring semi-urgent evaluation. It is important that GPs employ their clinical acumen in the referral process. For example, if there is concomitant vital sign instability or focal neurologic symptoms in conjunction with visual distortions, referral to an emergency department may be warranted for a holistic work-up. If there is uncertainty about the aetiology in a general practice setting, where diagnostics are limited, it is prudent to refer for further evaluation by an optometrist or ophthalmologist.
Key points
- Visual distortions can indicate a scope of macular pathologies, from benign to potentially sight-threatening.
- A directed history and examination using office-based techniques including visual acuity, Amsler grid and fundoscopy are helpful in the initial assessment.
- Urgency of ophthalmological evaluation is based on the nature of the presentation.