Otitis externa (swimmer’s ear) is an inflammation of the outer ear and ear canal. It is a common problem for which patients present to general practitioners (GPs), particularly in coastal temperate and tropical climates. Its monthly incidence in the USA increases during the summer season from 0.2% to 1.4% of the population.1 Otitis externa is more common in regular swimmers, compared with non-swimmers.2 There are no data for annual incidence in Australia. However, in tropical parts of Australia the annual incidence is likely to be much higher than 1.4% of the population.
The skin in the external ear canal of a healthy ear has a thin protective coating of cerumen, a mixture of secretions from apocrine and sebaceous glands mixed with desquamated epithelial cells. Interruption or alteration of this protective layer by trauma and/or exposure to moisture may result in inflammation. Many factors such as genetics (shape and size of ear canal, effectiveness of immune system, concomitant dermatological illness), environment (tropical climate), occupation (hearing protection and/or humid working conditions), recreational activities (water sports) and personal hygiene (use of ear buds, attempts at cleaning the ear canal with water) facilitate the development of such inflammation. An infectious organism cannot be found in at least one-third of patients with otitis externa.3 In the other two-thirds, it is not always clear if the identified organism is causing the signs and symptoms or is commensal. A secondary infection is likely in severe cases, and common organisms found are Pseudomonas spp., Staphylococcus aureus and various fungi.3
Common consequences for patients with otitis externa are pain, sleep disturbance, temporary loss of hearing, pharmaceutical and consultation expenses, and potentially loss of income. Initial symptoms at presentation to medical practices range from mild irritation with almost no pain to the strongest pain imaginable as measured by a pain scale.4 The average experience is slightly below ‘very strong pain’.4 The pain is often proportionate to the swelling in the ear canal, which, if canal closure occurs, also makes the condition very difficult to treat with topical medication. As well as pain, other consequences are costs for healthcare and sometimes also loss of productivity. Most patients fully recover after 5–14 days.4 In rare cases, severe damage to the pinna, outer ear and middle ear may result in significant hearing loss. Infection may also spread to deeper structures such as the inner ear and the brain, which can be potentially life-threatening.5
The treatment for otitis externa is usually topical; in selected cases, oral antibiotics are prescribed.6,7 In an Australian study of 201 patients, 95% received topical treatment and 30% received oral antibiotics.3 Topical treatment usually consists of antibiotics and corticosteroids.3 Rosenfeld et al performed a systematic review and reported that topical corticosteroids alone are equally effective as topical corticosteroids plus topical antibiotics.8 Roland et al found that topical treatment with a more powerful topical steroid might be more effective than topical treatment with a less potent topical steroid, even if the latter is combined with antibiotics.9–11
For many years, a published Australian ‘practice tip’ promoting use of oral corticosteroids for otitis externa has been practised by a small number of GPs.3,7,12 This tip recommends a short course of oral corticosteroids to reduce the swelling and gain control of pain and access to the canal for topical medication and cleaning.13 It has been anecdotally suggested that this results in faster pain relief, often dramatically so, and expedited recovery. Prednisone or prednisolone is used in doses ranging from 20 to 75 mg daily for 3–5 days.
Corticosteroids reduce the immune response. Therefore, corticosteroids given to a patient who has a severe infection could theoretically be detrimental. However, it has previously been shown that corticosteroids can be given safely and with beneficial effect to patients with ongoing infection of low or moderate virulence. Examples are patients with croup14 and sore throat.15–17
Acute otitis externa is in most cases either an aseptic inflammation that is simultaneously colonised by bacteria, or an infection of low-to-moderate virulence. In these situations, corticosteroids could theoretically be beneficial. Current evidence indicates that a topical steroid is beneficial to patients with otitis externa.11 It is therefore likely that otitis externa is rarely a highly virulent infection and that the risk of using an appropriate dose of oral corticosteroids is small.
We were unable to identify a published clinical trial evaluating the effect of oral corticosteroids in patients with otitis externa. Giving oral corticosteroids to patients with otitis externa could be beneficial or harmful. It may be that oral corticosteroids in the lower dose range are beneficial while using higher doses could add side effects and risks without benefit. If a short course of low-dose oral corticosteroids (20 mg prednisone daily) is beneficial, then this finding is useful for practitioners currently prescribing a higher dose. If a benefit of oral corticosteroids is not proven, then physicians currently prescribing it need to be advised of this finding.
The objective of this study was to assess the efficacy of low-dose oral prednisolone for four days in addition to conventional therapy in the management of painful acute otitis externa.
Methods
Study design and trial registration
This triple-blinded randomised controlled clinical trial was approved by the Human Research Ethics Committee (HREC)at James Cook University, Australia, (2014:C16) as well as the Australian Therapeutic Goods Administration (Trial Number: 2015/0442). It was registered at the Australian New Zealand Clinical Trials Registry with the registration number ACTRN12615000059561, and the full study protocol approved by the HREC (which has been adhered to) can be downloaded.18 The corresponding Universal Trial Number is U1111-1165-2370.
Study objectives
Primary research questions and subsequent data collection aimed to comply with the only published validated questionnaire for acute otitis externa.4 The primary research questions were:
- Will oral corticosteroids reduce time (number of days) to resolution of pain?
- Will oral corticosteroids reduce the number of ‘lost hours’ in respect to:
- need for bed rest
- activity limitation
- paid work missed
- need for paid child/elder care?
- Will oral corticosteroids increase patient satisfaction concerning:
- burning or stinging feeling post-administration of topical treatment
- itching post-administration of topical treatment
- time to resolution of pain
- time to resolution of itching
- time to resolution of swelling
- time to resolution of discharge?
Secondary research questions were:
- Will oral corticosteroids reduce the need for:
- unplanned revisits
- exclusion due to worsening of symptoms?
- Will Indigenous (Aboriginal and Torres Strait Islander) patients have the same outcome as Caucasian patients?
- Will oral corticosteroids increase patient satisfaction concerning time to resolution of normal activities?
- Will oral corticosteroids reduce time (number of days) to reduction of pain from at least ‘moderate pain’ to less than ‘moderate pain’?
Patients and recruitment
Sixteen primary healthcare centres and 19 adjacent pharmacies in tropical Far North Queensland, Australia, agreed to participate. Consecutive patients attending participating primary healthcare centres for otitis externa were asked by the medical practitioner if they accepted screening in relation to inclusion criteria:
- has pain at a level where visual analogue scale (VAS) is ≥2.5 cm of maximum 10 cm (ie at least ‘moderate pain’)
- will be staying in Australia for at least 10 days (not leaving the country within a few days)
- is aged ≥16 years
- is not pregnant
- has no cognitive impairment
- speaks English well enough to understand instructions and consent form
- has no large visual impairment that would preclude completion of the patient’s diary and questionnaire
- does not have Down syndrome
- does not have obvious craniofacial abnormalities
- does not have diabetes mellitus
- does not have known immunodeficiency (eg human immunodeficiency virus, leukaemia)
- is not taking immunosuppressant medications or oral corticosteroids
- does not have known rupture of the tympanic membrane
- does not have grommet (tympanostomy tube)
- does not have signs of systemic sepsis (body temperature >38.5 ˚C), invasive fungal disease or perichondritis of the pinna.
Patients fulfilling all inclusion criteria were referred to one of the participating pharmacies, where further information was given, consent forms were signed and the study medication was dispensed. A website was created as an ongoing resource for GPs and pharmacists (www.otitisexterna.net).19 This website also contained instructional videos for GPs and pharmacists. Furthermore, GP clinics and pharmacies were visited regularly to ensure they adhered to the agreed study protocol.
Data collection
Age, gender, ethnicity and initial ear pain was noted at baseline. Initial ear pain was measured using a VAS of 10 cm (Figure 1). The VAS, subsequent diary and final survey after symptom resolution or up to 10 days after enrolment adhered to the validated VAS, diary and survey published by Shikiar et al in 1999.4 Swabbing for potential microorganisms was not done as part of this study.
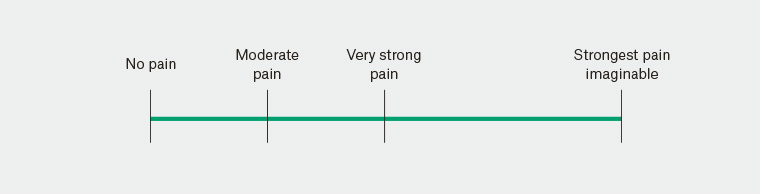
Figure 1. Visual analogue scale
Randomisation
Randomisation was achieved using random numbers generated by the ResearchRandomizer website
(www.randomizer.org), and sealed opaque envelopes.
Blinding
Medical practitioners, participating pharmacists, patients, staff telephoning patients and the person doing statistical analysis were all unaware of group allocation.
Intervention
The pharmacist checked inclusion criteria for a second time and provided study tablets to patients accepting participation. The intervention was a study capsule taken twice daily for four days in addition to any other treatment prescribed by the medical practitioner. Capsules with the active ingredient contained 10 mg of prednisone packed in an opaque gelatine capsule. The remaining space was filled with lactose. Capsules with placebo contained lactose packed in a gelatine capsule which was identical in appearance to capsules with the active ingredient. The lactose content was considered insignificant for patients with lactose intolerance.
Statistical analysis
All patients fulfilling inclusion criteria and with data available were analysed as follows:
- Time to resolution of pain: groups were compared using a log rank test. Cox regression was used in case clinically relevant baseline differences existed between groups. This was also done for the secondary research question of reducing pain from at least ‘moderate pain’ (≥2.5 cm on VAS) to below ‘moderate pain’ (<2.5 cm on VAS).
- Lost hours: the number of ‘lost hours’ per patient with respect to the need for bed rest, activity limitation, paid work missed and need for paid child/elder care was compared between groups using a Mann-Whitney U test. This test was chosen as the data were ordinal
- Satisfaction with symptom resolution: patient satisfaction was compared between groups using a Mann-Whitney U test.
- Secondary research questions such as unplanned visits and need for exclusion were analysed using two-tailed Fisher’s exact probability testing. Patient satisfaction was analysed using a Mann-Whitney U test.
The analysis was done as intention to treat. Intention to treat was defined as all patients fulfilling the inclusion criteria with follow-up data available, making analysis possible irrespective of whether they adhered to the allocated treatment arm. Imputation of data for patients lost to follow-up was not made. Patients were censored in the survival analysis at the end of the 10-day follow-up period if they had not achieved less than ‘moderate pain’ or ‘no pain’.
Sample size calculation
Sample size calculations were based on the primary research questions and made two-tailed to avoid the assumption that a difference between groups would always favour the intervention group. Sample size calculations for survival analysis used the statistical software PASS version 11.0.8.20 Other sample size calculations were done using the statistical software G*Power version 3.1.3.21,22
We calculated that 198 patients would be sufficient to answer all primary research questions. We expected that some patients would be lost to follow-up so we aimed to include 250 patients. A more detailed description of the sample size calculation is described in the full study protocol.18
Withdrawal from the study or discontinuation of the study
The following patient categories were instructed to be excluded and withdrawn:
- patients with moderate worsening of pain after taking two or more doses of the study tablets who still had more study tablets to take
- patients with severe worsening of pain while still taking the study tablet
- patients with fever >38.5˚C while still taking the study tablet
- other types of adverse events, evaluated on a case-by-case basis as needed.
Patients with any type of side effect mentioned above were instructed in the written information to immediately contact their GP (or nearest emergency department if their GP was unavailable). The patient information also outlined that those patients must immediately stop taking the study tablets. Furthermore, they were instructed to notify the steering committee. Patients were also withdrawn from the study if it was their wish. Detailed rules for discontinuation of the study are presented in the study protocol.18
Role of the funding source
The funder, Cairns Hospital Foundation, did not participate in planning, analysing data or writing of the manuscript.
Patient and public involvement
Patients or the public were not involved in the design of this study.
Steering committee
The steering committee comprised Peter Morris (MBBS, FRACP, PhD, Professor in Paediatrics at Menzies School of Health research [chair]), Malcolm McDonald (MBBS, PhD, Infectious Disease Specialist and Associate Professor at James Cook University) and Ronny Gunnarsson (MBBS, PhD and adjunct Professor in General Practice, Public Health and Community Medicine, Institute of Medicine, the Sahlgrenska Academy, University of Gothenburg, Sweden).
Results
One hundred and sixty-four patients were screened for eligibility between 28 October 2015 and 19 June 2017. Seventy-three patients were randomised and given instructions with surveys to return and a can containing the study tablets. Forty-three of these patients could not be analysed, while 30 patients submitted identifiable surveys and were included in the final analysis (Figure 2). The proportion of male gender was 29/43 (67%) among the 43 patients not included in the final analysis and 23/30 (77%) among the 30 patients included in the final analysis (P = 0.44, two-tailed chi-squared test). The mean age was 41 years (standard deviation [SD]: 13 years) among the 43 patients not included in the final analysis and 52 years (SD: 15 years) among the 30 patients included in the final analysis (P = 0.003, two-tailed Student’s t-test).
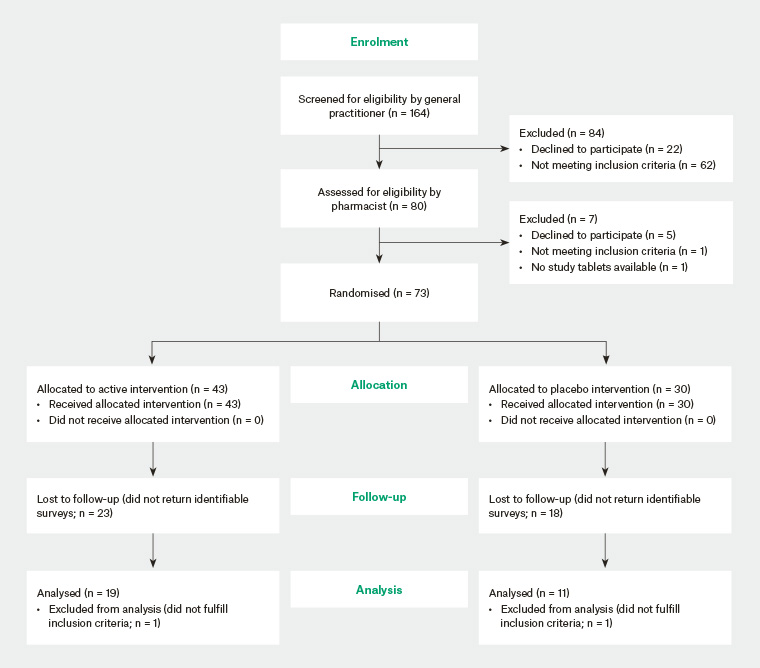
Figure 2. CONSORT flow diagram
This study did not find evidence that the intervention and control groups differed statistically at baseline (Table 1). Two patients in the intervention group stated they took only 3–4 out of eight study tablets. No reason for this was given. All other patients included in the final analysis stated they took all eight study tablets.
| Table 1. Baseline characteristics for patients included in the final analysis |
| |
Intervention group
n = 19 |
Control group
n = 11 |
P value* |
| Male gender (%) |
16 (84%) |
7 (64%) |
0.37 |
| Age in years (SD) |
55 (11) |
44 (20) |
0.19 |
| Ethnicity |
|
|
|
| Aboriginal or Torres Strait Islander |
1 |
0 |
0.62 |
| Caucasian |
13 |
9 |
| Other |
5 |
2 |
| Swelling of ear canal |
|
|
|
| None |
5 |
3 |
0.46 |
| Some |
12 |
5 |
| Occluded ear canal |
2 |
3 |
| Inflammation and/or redness in ear canal (%) |
16 (84%) |
11 (100%) |
0.28 |
| Discharge from ear canal (%) |
12 (63%) |
9 (82%) |
0.28 |
| Tender subauricular lymph nodes (%) |
7 (37%) |
5 (45%) |
0.54 |
| Current pain on VAS in cm† |
|
|
|
| Mean (SD) |
3.7 (1.5) |
4.5 (1.7) |
0.20 |
| Median (min–max) |
3.0 (2.5–8.0) |
4.3 (2.5–7.4) |
0.25 |
| Any initial cleaning of ear canal |
6 (32%) |
3 (27%) |
1.0 |
*Difference between groups for binary variables analysed with chi-squared tests and, in case of small numbers, Fisher’s exact test.
†Student’s t-test was used for comparing means; Mann-Whitney U test was used for comparing medians.
SD, standard deviation; VAS, visual analogue scale |
Primary research questions
It took an average of 5.5 days for the control group and 5.2 days for the intervention group to be pain-free (P = 0.77, log rank test; hazard ratio [HR]: 0.80; 95% confidence interval [CI]: 0.36, 1.8; P = 0.58, Cox regression adjusting for baseline pain; Table 2). Lost hours as a result of otitis externa were similar in both groups (Table 2). Side effects during treatment were expected and similar in both groups (Table 3).
| Table 2. Hours lost as a result of otitis externa and time to pain resolution for patients included in the final analysis |
| |
Intervention group
n = 19 |
Control group
n = 11 |
P value* |
| Hours of bed rest (n = 29) |
|
|
|
| Mean (SD) |
2.4 (4.3) |
4.8 (3.6) |
0.14 |
| Median (IQR) |
0.0 (0.0–3.0) |
5.0 (1.0–7.0) |
0.062 |
| Hours of activity limitation (n = 30) |
|
|
|
| Mean (SD) |
3.6 (5.4) |
6.9 (8.0) |
0.18 |
| Median (IQR) |
2.0 (0.0–4.0) |
3.0 (0.0–14) |
0.42 |
| Hours of paid work lost (n = 29) |
|
|
|
| Mean (SD) |
2.3 (5.8) |
1.0 (2.2) |
0.49 |
| Median (IQR) |
0.0 (0.0–2.0) |
0.0 (0.0–0.0) |
0.77 |
| Hours of paid child/elder care required (n = 29) |
|
|
|
| Mean (SD) |
0.36 (1.6) |
0.40 (1.3) |
0.95 |
| Median (IQR) |
0.0 (0.0–0.0) |
0.0 (0.0–0.0) |
0.88 |
| Follow-up time until less than ‘moderate pain’ (days) |
|
|
|
| Mean (SD) |
2.4 (1.1) |
3.7 (1.3) |
0.0067 |
| Median (IQR) |
2 (1–3) |
4 (3–5) |
0.0086 |
| Follow-up time until ‘no pain‘ (days) |
|
|
|
| Mean (SD) |
5.2 (2.1) |
5.5 (1.8) |
0.75 |
| Median (IQR) |
5 (3–7) |
5 (4–7) |
1.0 |
*Student’s t-test was used for comparing means; Mann-Whitney U test was used for comparing medians.
IQR, interquartile range; SD, standard deviation |
Secondary research questions
Unscheduled revisits to the doctor occurred for 3/19 patients in the intervention group and 1/11 patients in the control group (P = 1.0, two-tailed Fisher’s exact probability test). None of these revisits were considered unexpected or serious, and all four patients became completely pain-free in an average of 4.8 days (the mean for all 30 patients was 5.3 days). No patient was excluded as a result of worsening of symptoms. The influence of ethnicity was not analysed because most patients were of Caucasian ethnicity (Table 1). Patient satisfaction after treatment was similar in both groups (Table 3).
| Table 3. Side effects during treatment and patient satisfaction after treatment for patients included in the final analysis |
| |
Intervention group
n = 19 |
Control group
n = 11 |
P value* |
| Side effects: Burning or stinging feeling post-administration of topical treatment (n = 9) |
|
|
|
| Mean (SD) |
3.0 (1.0) |
5.0 (1.0) |
0.080 |
| Median (IQR) |
3 (2–4) |
5 (4–5) |
0.095 |
| Side effects: Itching post administration of topical treatment (n = 20) |
|
|
|
| Mean (SD) |
4.0 (1.0) |
4.0 (1.0) |
0.69 |
| Median (IQR) |
4 (3–4) |
4 (4–4) |
0.69 |
| Satisfaction with time to resolution of ear pain (n = 26) |
|
|
|
| Mean (SD) |
4.0 (2.0) |
4.0 (1.0) |
0.32 |
| Median (IQR) |
4 (2–5) |
5 (3–5) |
0.43 |
| Satisfaction with time to resolution of itching (n = 18) |
|
|
|
| Mean (SD) |
4.0 (1.0) |
4.0 (1.0) |
0.57 |
| Median (IQR) |
4 (3–5) |
4 (3–4) |
0.49 |
| Satisfaction with time to resolution of swelling (n = 22) |
|
|
|
| Mean (SD) |
3.0 (2.0) |
3.0 (1.0) |
0.99 |
| Median (IQR) |
4 (2–5) |
4 (3–4) |
0.86 |
| Satisfaction with time to resolution of discharge (n = 9) |
|
|
|
| Mean (SD) |
4.0 (1.0) |
4.0 (1.0) |
0.95 |
| Median (IQR) |
3 (3–5) |
4 (3–5) |
1.0 |
| Satisfaction with time to resume normal activities (n = 28) |
|
|
|
| Mean (SD) |
4.0 (1.0) |
4.0 (1.0) |
0.56 |
| Median (IQR) |
5 (4–5) |
4 (4–5) |
0.52 |
*Student’s t-test was used for comparing means; Mann-Whitney U test was used for comparing medians.
IQR, interquartile range; SD, standard deviation |
It took an average of 3.7 days for the control group and 2.4 days for the intervention group to have pain fall below 2.5 cm (‘moderate pain’) on the VAS (P = 0.012, log rank test; HR: 0.42;
95% CI: 0.18, 0.99; P = 0.047, Cox regression adjusting for baseline pain; Table 2).
Discussion
The main finding was that oral corticosteroids reduced the time to reporting having less than ‘moderate pain’ from 3.7 days to 2.4 days. However, oral corticosteroids did not reduce the time to reporting being completely pain-free (complete resolution of pain). Therefore, this trial suggests that oral corticosteroids are mainly effective when patients have greater than ‘moderate pain’ on a VAS (ie the more severe or strong end of the pain spectrum).
Limitations
The main limitations of this study were recruitment of participants and loss to follow-up of included participants. Recruitment was slower than anticipated, and fewer than half of the patients who were screened were suitable for inclusion. The target was never reached: after 20 months of recruiting, the study was terminated because of slow recruitment of patients. Fewer than half of the randomised patients returned identifiable surveys. The following potential problems were identified:
- The researchers noted that the wet seasons in 2015–16 and 2016–17 were unusually dry, resulting in fewer than expected cases of otitis externa. Many GPs in the participating clinics also expressed there were fewer cases than usual.
- There is always a time pressure in primary healthcare, and actions linked with financial remuneration are often given some priority. Remuneration for participating practitioners, pharmacists or patients was not available in this study because of the limited funding allocated. After discussions with colleagues, the researchers first believed recruitment would work well without remuneration. Afterwards, it became evident that this assumption was incorrect, and remuneration to medical practitioners and pharmacists for each included patient, and a small remuneration to patients for returned surveys, may have reduced the recruitment problem and loss to follow-up.23 Lack of remuneration is considered to have been the main problem.
- Each can containing study tablets had a unique identifying number, which pharmacists were instructed to note on the survey handed out to patients. Many pharmacists failed to do so, and these returned surveys could therefore not be linked with the correct patient. A checklist was introduced for pharmacists halfway through the study, and this problem was significantly reduced.
A formal process evaluation24 to see if further lessons could be learnt was not done because of lack of funding. Clinical follow-up by the medical practitioner on days three and six would have added useful information. However, this would have required substantial funding that was not available.
Generalisability
This study was planned as a randomised controlled trial (RCT) but, most likely because of insufficient funding, failed to recruit enough patients to be adequately powered to assess the proposed outcomes. However, the study indicates that the measuring tools worked well, the intervention was accepted by patients and the sample size calculation is likely to be adequate. Although we did not plan this to be a pilot study, and it should be classified as an underpowered RCT, our outcomes are useful to inform a larger study in a similar manner to a pilot study. Therefore, these potentially interesting results should be confirmed in a larger, properly funded clinical trial before applying the results in the routine healthcare setting.
Conclusions and implications for general practice
Oral corticosteroids seem to be effective in reducing more than ‘moderate pain’ to less than ‘moderate pain’. However, the effect on patients with less than ‘moderate pain’ is smaller, and this study was not powered to clarify whether the effect in these patients is clinically or statistically significant. Shortening the duration of intense pain by 1.3 days by using a very cheap intervention seems cost-effective and relevant from the patient perspective. Therefore, pursuing this research with a follow-up study adequately powered to measure complete resolution of pain as an outcome makes sense. However, for a larger study to be feasible, reasonable funding for reimbursement for healthcare providers and participating patients is likely to be required. A future study with a larger number of patients available for statistical analysis could also investigate the extent to which the effect of oral corticosteroids is influenced by baseline pain, sleep disturbance due to symptoms, occlusion of the ear canal or initial cleaning of the ear canal using suction under microscope.