When women with symptoms present around the time of menopause, it provides an opportunity for clinicians to assess and promote health. Both the clinician and patient face choices about hormonal and other treatments to address symptoms and long-term health. Although menopausal hormone therapy (MHT) is by far the most effective treatment for menopausal symptoms, there has been conflicting information about its safety. Advances in the understanding of the evidence regarding the benefits and risks of MHT mean that clinicians can have the confidence to prescribe MHT safely to women with no contraindications, and adjust the type and delivery of MHT for those who do have relative contraindications. Choices relating to the timing and types of treatments are important to optimise benefit and minimise risk. Women need clear information to make decisions that are appropriate for their circumstances.
What is menopause?
Menopause is caused by cessation of ovulation and is defined as the final menstrual period.1 For Australian women, the average age at menopause is approximately 51 years, with a normal range of 45–55 years. Women who have their final period between the ages of 40 and 45 years have early menopause, and those aged under 40 years have premature menopause, or premature ovarian insufficiency. Prior to the final period, women have a phase of fluctuating ovarian function and hormone levels known as perimenopause, which typically lasts several years.
Menopause results in a number of physiological changes affecting the cardiovascular, musculoskeletal, urogenital and central nervous systems. There is an increase in incidence of cardiovascular disease and osteoporosis after menopause.
During the perimenopausal and postmenopausal years, women often have a variety of symptoms including vasomotor symptoms (hot flushes and night sweats), muscle and joint pains, sleep disturbance, mood changes and anxiety, and genitourinary symptoms. These symptoms are often more pronounced in women with premature menopause or menopause due to surgery or other medical interventions.
The menopause consultation
The clinical management of a women who is perimenopausal or menopausal necessitates a comprehensive assessment and a detailed discussion of treatment options, and this may require several consultations.2 Women may present with a range of symptoms and concerns. The consultation provides an opportunity to explore these concerns, along with a review of personal and family medical history, with emphasis on venous thromboembolism (VTE), cardiovascular disease and risk factors, cancer and osteoporosis. This is also an opportune time to ensure that mammography, cervical screening and bowel cancer screening are up to date.
A discussion about menopause provides an opportunity for health promotion: smoking cessation, healthy eating, exercise and minimising alcohol intake are all important.
Measurement of follicle-stimulating hormone is not necessary for women at the normal age for menopause. Blood tests are appropriate in women under 45 years of age if early or premature menopause is suspected, and to exclude other causes of oligomenorrhoea or amenorrhoea. Women should be asked about contraceptive needs. Women aged under 50 years should be offered contraception for two years after the final menstrual period, and women aged 50 years and over should be offered contraception for one year after the final menstrual period, although the risk in the latter group is small.3
Treatment options at menopause
For women with symptoms, treatment options fall into three categories: MHT, non-hormonal prescription treatments and complementary therapies. The most effective treatment for menopausal symptoms is MHT, and it can be offered to all symptomatic women who do not have contraindications (Box 1).
| Box 1. Contraindications to menopausal hormone therapy |
Contraindications to menopausal hormone therapy (MHT):
- breast, endometrial and other hormone-dependent cancers (current or previous)
- undiagnosed vaginal bleeding.
Conditions that are relative contraindications when transdermal MHT is preferred:
- established cardiovascular disease
- venous thromboembolic disease13
- active liver disease
- possibly migraine with aura.
Note that treated hypertension is not a contraindication. |
Benefits and risks of menopausal hormone therapy
The Women’s Health Initiative (WHI) trial, first reported in 2002,4 has had an ongoing impact on the perception of the risks of MHT among both clinicians and the general public, with many perceiving MHT as an unsafe treatment, especially with respect to breast cancer risk. The WHI trial reported increased risks of breast cancer, cardiovascular disease and VTE in participants using MHT. Subsequent re-analyses of the WHI data, and other randomised trials and observational studies, have led to a change in our understanding of the benefits and risks of MHT.5,6
National and international menopause organisations now advise that the reports of harm attributed to MHT were overstated. In addition, the WHI trial findings have limited applicability to women with symptoms who are making a decision about whether to start MHT.1,7,8 Participants in the randomised controlled component of WHI had a mean age of 63 years (with a range of 50–79 years). Despite the known differences in effects of oestrogen treatment in different age groups, claims were made that the trial findings of harm, particularly cardiovascular harm, applied to all women irrespective of age. However, in the 2017 publication of long-term outcomes of WHI participants, there was no difference in the rate of all-cause mortality between women randomised to MHT or placebo; women aged 50–59 years who were randomised to MHT had lower all-cause mortality during the study (hazard ratio: 0.69, 95% confidence interval [CI]: 0.51, 0.94).9 With respect to fracture risk, a meta-analysis has shown that MHT is associated with a 34% reduction in risk of vertebral fracture, a 29% reduction in hip fracture and a 21% reduction in non-vertebral fracture when compared with placebo.10
Advice from the International Menopause Society is that the use of MHT is considered safe when initiated before the age of 60 years in women with symptoms who do not have any contraindications.1
In light of the confusion and controversy surrounding the risks of MHT, it is beneficial to discuss these risks with women in a systematic way.
1. Breast cancer
This is often the main concern for women. In the combined oestrogen–progestogen arm of the WHI study, there was an increase in breast cancer incidence that equates to an absolute increase of fewer than one case per 1000 women per year of use, including with long-term use. For women taking oestrogen only, there was a decrease in the incidence of breast cancer. These data highlight that it is the addition of progestogen that adds risk. Furthermore, epidemiological studies suggest that other progestogens, specifically micronised progesterone and dydrogesterone, may be associated with a lower risk than medroxyprogesterone acetate.11 For women with a high risk of breast cancer, even for those with BRCA mutations, there is no evidence of a greater increase in risk with MHT than that observed with MHT in the general population.12
2. Cardiovascular disease
There is no increase in cardiovascular disease in young women who are perimenopausal or postmenopausal in the absence of established cardiovascular disease. For women who start MHT within 10 years of menopause or before the age of 60 years, coronary heart disease (composite of death from cardiovascular causes and non-fatal myocardial infarction) is reduced by 48% (risk ratio: 0.52, 95% CI: 0.29, 0.96; absolute difference: 7 per 1000 women). There is no significant increase in stroke in this group of women.13
3. Venous thromboembolism
The risk of VTE is increased by smoking, increasing age, obesity and oral MHT.14 The use of transdermal MHT has not been associated with increased VTE risk and is recommended for women with risk factors for VTE or previous VTE, provoked or unprovoked, with or without an identified thrombophilia.15
How to prescribe MHT
Once a decision has been made to prescribe MHT, the clinician has a number of choices regarding the type and route of MHT (Figure 1). The Australasian Menopause Society (AMS) provides information about available MHT preparations, along with guidance about other aspects of menopause management.16
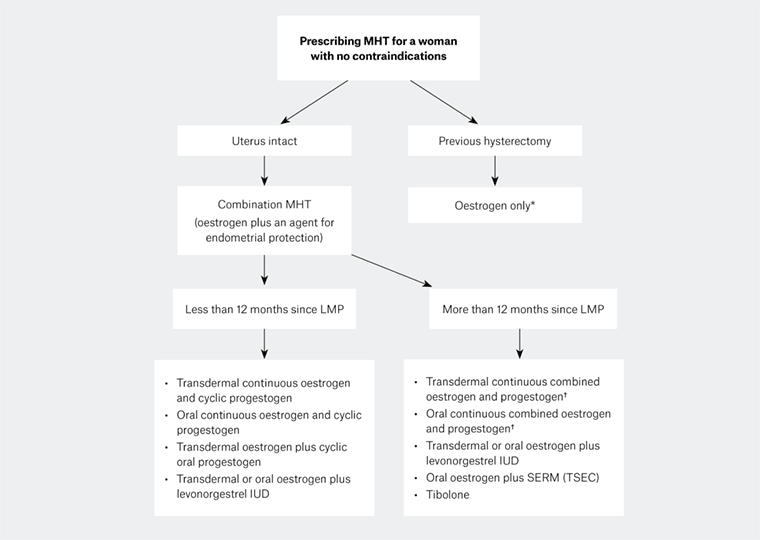
Figure 1. Prescribing menopausal hormone therapy
*Possible exception: women who have residual endometriosis after hysterectomy
†Alternatively, women can continue continuous oestrogen and cyclic progestogen
IUD, intrauterine device; LMP, last menstrual period; MHT, menopausal hormone therapy; SERM, selective oestrogen receptor modulator; TSEC, tissue-selective estrogen complex
MHT comprises continuous oestrogen for symptom management and other physiological effects, with a progestogen added for endometrial protection for women who have not had a hysterectomy. For women who had their last menstrual period less than 12 months ago, cyclical MHT (continuous oestrogen with cyclic progestogen) is recommended, because using continuous combined oestrogen and progestogen can result in irregular breakthrough bleeding in these women. If desired, this can be changed to continuous combined MHT after 12 months. Some women may continue to have irregular bleeding on continuous treatment and may require another year of cyclical MHT. Ongoing breakthrough, irregular or heavy bleeding after six months on continuous combined therapy must be investigated according to established guidelines.17
Oral and transdermal MHT options are available, with the choice depending on risk profile and patient preference. For example, women with known risk factors for VTE or established cardiovascular disease, or those with a history of migraine, may benefit from using transdermal oestrogen. Oral and transdermal options are available on the Pharmaceutical Benefits Scheme with no restrictions. A list of available options for prescription in Australia can be found on the AMS website.16
After commencing MHT, women should be reviewed, typically at 6–12 weeks, and treatment individually tailored according to efficacy and side effects. For example, if vasomotor symptoms are not adequately controlled, the dose of oestrogen can be increased, but if troublesome breast tenderness occurs, the dose can be reduced. If adequate cycle control is not achieved on a cyclic regimen (and after assessing whether further investigation is needed), the dose of progestogen can be increased or intrauterine progestogen administration can be considered.
Duration of treatment
Once the formulation and dose of MHT is established, reviews should occur at least annually to check symptom control and for the emergence of contraindications. The International Menopause Society recommends that, contrary to advice found in MHT product information, there is no need for a mandatory limit on the duration of MHT (Box 2).1 In fact, a recent data linkage study has found that women who stopped MHT had a significant increase in cardiovascular and cerebrovascular death when compared both with the general population and with those who continued MHT.18 More than 20% of women will have moderate-to-severe symptoms after discontinuing MHT.19 In the absence of contraindications, women can continue to take MHT for as long as it addresses treatment goals, which can be either symptom relief or long-term health such as the maintenance of bone density. The majority of women will have menopausal symptoms for up to eight years, and 20% will have symptoms into their 60s and 70s.20 Of course, the bone, genitourinary and cardiovascular consequences of oestrogen deficiency continue long term. Over time, the dose, type of MHT and mode of delivery may be adjusted.
| Box 2. Duration of treatment with menopausal hormone therapy |
- The duration of treatment with menopausal hormone therapy (MHT) should be determined on an individual basis.
- There is no mandatory duration of therapy in the absence of emerging contraindications.
- In epidemiological studies, cessation of MHT has been associated with an increase in vascular death.
- The mode of delivery and the dose may be adjusted over time.1
|
For women who have an early or premature menopause, MHT is recommended until at least 51 years of age unless there are contraindications. Thereafter, a shared decision about whether to continue MHT can be made in the same way as it would be for other women of this age.
Choosing non-hormonal medications
Women with contraindications to MHT, or those who prefer not to use hormones, may choose to use non-hormonal medicines for relief of vasomotor symptoms. These are not as effective as MHT, but some women have a clinically significant reduction in symptoms. The serotonin–noradrenaline reuptake inhibitor (SNRI) antidepressants venlafaxine and desvenlafaxine and the selective serotonin reuptake inhibitors (SSRIs) escitalopram and paroxetine all have evidence of benefit.21 Four weeks of SSRI or SNRI treatment should be sufficient to establish whether the treatment is effective. These medications are used at low-to-moderate doses for vasomotor symptoms; higher doses may cause sweating. Table 1 lists the doses and side effects of non-hormonal medications.
There is evidence of benefit with gabapentin, and pregabalin is also used, though evidence of efficacy is weaker for pregabalin than gabapentin. There is also evidence of benefit for the use of clonidine, though its use is sometimes limited by adverse effects.16
Apart from clonidine, which is approved by the Therapeutic Goods Administration for use for hot flushes, the use of non-hormonal medicines for menopausal symptoms is ‘off-label’ in Australia. Non-hormonal medications do not generally relieve menopausal symptoms other than vasomotor symptoms and do not have the potential benefits for bone and cardiovascular health that MHT has.
| Table 1. Non-hormonal medications for treatment of menopausal vasomotor symptoms21 |
| Medication |
Suggested dosage |
Common adverse effects |
| SSRIs and SNRIs |
|
|
| Escitalopram |
10–20 mg/day |
Nausea, drowsiness, sexual dysfunction* |
| Paroxetine† |
10–20 mg/day |
| Venlafaxine |
37.5–150 mg/day |
| Desvenlafaxine |
25–150 mg/day |
| Other medications |
|
|
| Gabapentin |
100 mg at night, slowly titrating to maximum 300 mg three times per day |
Dizziness, drowsiness |
| Pregabalin |
75 mg at night, slowly titrating to maximum 150 mg twice daily |
Dizziness, drowsiness, nausea, headache |
| Clonidine |
25 mcg twice daily, slowly titrating to maximum 75 mcg twice daily |
Dry mouth, drowsiness, visual disturbance |
*Adverse effects vary between antidepressants and between patients, so a trial of more than one medication may be helpful.
†Do not prescribe paroxetine to women taking tamoxifen.31
SSRI, selective serotonin reuptake inhibitors; SNRIs, serotonin–noradrenaline reuptake inhibitors |
Choosing complementary therapies
There are very few complementary therapies with any evidence for efficacy. Both cognitive behavioural therapy and clinical hypnosis have some evidence of efficacy for vasomotor symptoms.1,22 There is limited evidence for the use of soy isoflavone dietary supplements, mindfulness techniques and stellate ganglion blockade.18
There is no evidence for efficacy of herbal supplements, acupuncture, chiropractic or homeopathy.
Compounded ‘bio-identical’ hormones
Following the publication of the WHI trial data, there was a large and sustained drop in the prescription of MHT.23 Since this time, many women have been prescribed compounded ‘bio-identical’ products while avoiding regulated pharmaceutical hormones. Bio-identical products are heavily promoted as being ‘natural’ and therefore safe. They are often accompanied by expensive salivary hormone tests that have not been proven to provide accurate data. Unfortunately, the compounded hormone products lack safety and efficacy data, and are not recommended.24 Women who are seeking to use hormones that are identical to those produced by the ovaries can use prescribed oestradiol along with micronised progesterone.
Vulvovaginal and urinary symptoms
Vaginal dryness is a common symptom but is often not reported by women unless clinicians ask about it. Topical vaginal oestrogen therapy is useful, and non-hormonal options such as lubricants may also assist. Oestrogen may also reduce the incidence of urinary frequency and urinary tract infections. Women who have genitourinary symptoms without systemic menopausal symptoms can be prescribed topical oestrogen. Women who take MHT for systemic symptoms often find that it helps urogenital symptoms. However, some women require additional topical oestrogen.
The safety of long-term administration of unopposed vaginal oestrogen, with respect to the endometrium, lacks sufficient evidence to be totally reassuring. Many studies of vaginal oestrogen administration did not assess endometrial response, and most were short term. Nevertheless, an increase in endometrial thickness was reported in some studies, depending on dose and mode of delivery.25 Therefore, if vaginal oestrogen administration is used long term, endometrial surveillance and/or progestogen challenge should be considered.
For women with a history of breast or other hormone-dependent cancer who do not respond to non-hormonal options, it is recommended to discuss proposed vaginal oestrogen treatment with the treating oncologist.
Vaginal laser treatment is increasingly being promoted as a remedy for vaginal dryness and dyspareunia. There is currently no trial evidence for the safety and efficacy of this treatment, though a randomised controlled trial is underway in Australia at the time of publication. Until evidence of benefit is established, it is not recommended.26 Many women who seek vaginal laser treatment may benefit from topical oestrogen treatment, which is an established and cost-effective treatment.
Future directions in menopause management
In recent years, it has been increasingly recognised that different progestogens have differing physiological effects and may have varying effects on the risk–benefit profile of MHT.12,27 There is ongoing research into progestogens that may be safer for the breast, such as micronised progesterone and dydrogesterone, and into using selective oestrogen receptor modulators instead of progestogens for endometrial protection.28
There is considerable interest in the development of new non-hormonal treatments for menopausal symptoms. Following the discovery that neurokinin B neurons are involved in the aetiology of the hot flush, early research has found that neurokinin B receptor antagonists reduce the frequency and severity of hot flushes.29,30 Larger trials are needed before these options can be made available to patients.
Conclusion
Menopause is a time of significant change for women and an opportune time to assess and promote health. A range of treatment options are available for women who have symptoms. For women to make an informed choice about whether to use MHT, it is important for them to receive balanced and accurate information about the benefits and risks. Additional treatment options are likely to be available in the future.
Key points
- Menopause is a time of change for women and provides an opportunity for health assessment and promotion.
- MHT is the most effective treatment for symptom relief and can be offered to most women.
- Topical vaginal oestrogen treatment is effective in relieving urogenital symptoms.
- For women with contraindications to MHT or those who prefer not to take hormones, non-hormonal options are available, and new non-hormonal options are currently under investigation.