Over recent decades, there has been a steady increase in the incidence rates of melanoma reported in most western countries, although proportionate increases in mortality rates have not been observed.1,2 While this discrepancy may be partly attributed to a greater number of melanomas being diagnosed at an early clinical stage, leading to more favourable outcomes, it has been suggested that an important cause of rising incidence rates is overdiagnosis of benign lesions as melanomas by pathologists.3 There are a number of well-described clinical scenarios that cause benign melanocytic lesions to closely resemble melanomas when examined with a microscope, and these represent diagnostic traps.4–6 Clinicians may be contributing to overdiagnosis by not providing to the pathologist pertinent clinical information that would trigger the recognition of benign melanocytic lesions that can appear similar to melanomas. In many cases, the provision of relevant clinical information is also critical for the accurate pathological diagnosis of melanoma; without it, some melanomas are at risk of being misdiagnosed as benign naevi.6,7 This is not simply a theoretical concern. It is a sobering fact that little or no useful clinical information was recorded on 46% of pathology request forms from a random sample of 1200 cases of invasive melanoma reported to the Victorian Cancer Registry during a 10-year period (de Menezes and Mar, unpublished data), making it clear that communication of clinical information between treating clinicians and pathologists must be greatly improved. Misdiagnosis of melanoma may lead not only to inappropriate treatment, poor clinical outcomes and increased cost to the healthcare budget, but it can also have serious medicolegal consequences.8
The accuracy of any histopathology report is at least partly dependent not only on the amount of tissue provided, but also on the availability of relevant clinical details. Some of this clinical information may be sought and provided in generic pathology request forms; however, there is also specific additional clinical information required by the pathologist for the accurate diagnosis and optimal reporting of cutaneous melanocytic tumours.
This article, based on the recently revised Australian clinical practice guidelines for the diagnosis and management of melanoma,9 describes the clinical information required by pathologists to assist them to accurately diagnose melanocytic skin tumours. It highlights the need for close clinician/pathologist interaction to achieve more accurate diagnosis of melanocytic skin tumours, in turn allowing appropriate management.
What clinical information should be provided for melanocytic skin tumours?
In addition to the patient demographic details included on generic pathology request forms, the list of items of clinical information that may assist pathologists when interpreting specimens of possible melanoma is extensive (Table 1).
Several studies have shown that the inter-observer reproducibility for the pathological diagnosis of melanocytic tumours is increased, without introducing bias, when clinical information is provided to the pathologist.
10,11 Furthermore, it has also been shown that the histopathological diagnosis may change when appropriate clinical information is provided.
11
| Table 1. Clinical information that may aid pathologists in the diagnosis of melanocytic tumours of the skin |
| Clinical factor |
Information required |
Comments |
| Patient demographics |
Age, sex, ethnicity |
|
| Specimen type |
Excision
Punch
Incision
Shave
Curette
Re-excision
Not provided
Other |
If ‘other’ is selected, record the other specimen type.
It is also often useful if the intent of the biopsy is also recorded (eg a shave biopsy versus an attempted shave excision). |
| For re-excision specimens |
Previous laboratory report
Previous laboratory accession number
Findings in previous biopsy |
A copy of, or access to, the pathology report for the previous biopsy or excision is often the most practical method to provide the required information. Alternatively, important findings of the previous biopsy may be provided on the pathology request form (eg tumour thickness, ulceration, mitotic rate, desmoplastic type, positive surgical margins). |
| Specimen site and laterality |
Left/right |
|
| Details of specimen orientation (if appropriate) |
|
Excision specimens should be orientated if the status of specific surgical margins is (or might become) critical in determining the need for, or the extent of, further surgery. Specimen orientation may be indicated with marking sutures or other techniques.22 A diagram or provision of a photograph may be used to indicate the orientation. |
| Clinical diagnosis or differential diagnosis |
Text |
|
| Clinical reason for the biopsy |
Text |
Clinical suspicion or cosmetic/patient request |
| History of current lesion |
Text |
Duration, history or duration/rate of change, size of lesion, ulceration, any focally suspicious areas within the lesion (including apparent regression) |
| The history and timing of lesional trauma, biopsy, irritation or treatment with topical agent, laser or radiation therapy |
Details |
Many histopathological features that commonly occur in melanomas may occur in naevi that have undergone trauma, previous biopsy, irritation or topical treatment. These naevi may be over-diagnosed as melanoma unless the clinical context is known to the pathologist. |
| Is there a past history of melanoma? |
Details |
Site, thickness, timing, treatment, previous metastasis |
| Is there evidence of current or previous metastatic disease? |
Yes/no |
If yes, when and where and consider recording the serum lactate dehydrogenase for patients with stage IV disease |
| Other relevant history |
Text |
Family history of melanoma or dysplastic naevus syndrome, current or recent pregnancy, neurofibromatosis, personal or family history of p16 or BAP1 tumour predisposition syndromes |
| Are there any clinically or dermoscopically suspicious areas? |
Yes/no |
A diagram or clinical photograph may assist |
| Clinical/dermoscopy or other relevant diagnostic imaging results |
|
|
| New primary melanoma or recurrence |
New primary
Recurrence – local
Recurrence – in-transit metastasis (between primary site and regional node field)
Recurrence – regional
Recurrence – distant |
|
The most relevant clinical information
Clinical information particularly relevant to the diagnosis of melanocytic skin lesions includes patient age and gender, and the anatomical site of the lesion, as well as clinical and/or dermoscopic images.1,4,11,12 The diagnostic significance of any individual atypical pathological feature varies with the age of the patient and the site of the lesion. For example, naevi occurring on certain sites (including the palms, sole, breast, genitalia, ear and flexural sites) often display irregular architecture (ie asymmetry, predominance of single-cell growth and focal pagetoid migration) that would usually be considered evidence favouring melanoma in pigmented tumours occurring elsewhere.6,12,13 The presence of mitotic activity in a melanocytic lesion in a young child would be compatible with a Spitz naevus, but the same frequency of mitoses in an elderly patient would usually signify melanoma.14–16
The importance of the history of a lesion
It is particularly important that clinicians use the pathology request form to record factors that may induce atypical pathological features in melanocytic naevi (eg previous biopsy, topical treatment, trauma, laser or radiation therapy, surface irritation, pregnancy, recent prolonged sunlight exposure or sunburn episodes) and that may lead to an overdiagnosis of melanoma.4,17 Following lesional trauma, biopsy, topical treatment or irritation, naevi may display many of the histopathological features that commonly occur in melanomas (Figure 1).4,18 Atypical features typically occur within six months of a previous injury, and the atypical pathological changes are usually confined to the area affected by the inciting agent, whereas recurrent melanoma generally grows into the surrounding tissue.12,19 This may be a ‘portion’ of a naevus in the case of trauma/irritation/biopsy, but it may also be the entire lesion in the case of topical treatment (or even trauma/irritation). Since the histopathological changes of naevi or melanoma recurring after trauma may be very similar, it is essential that the previous biopsy and, if available, any relevant clinical and dermoscopic photographs be reviewed in conjunction with the clinician (Figure 2). Another important consequence of trauma is that it may result in ulceration. Therefore, in most cases of re-excision of melanoma it is difficult to determine whether such ulceration is ‘spontaneous’ and should therefore be considered as a negative prognostic factor, or whether it is traumatic (not ‘spontaneous’) and should therefore be ignored.20 More information on re-excision specimens is provided later in this article.
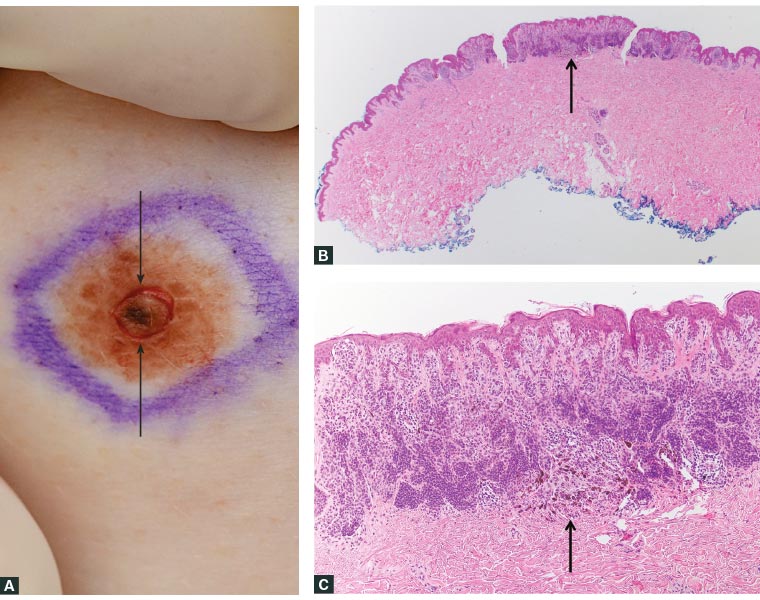
Figure 1. Scoring the area of concern within a lesion
A. A new focus of increased pigmentation developed within this longstanding melanocytic lesion. The surgeon scored the area of concern with a punch (circular defect, indicated by arrows) to assist the pathologist to identify it microscopically; B. Microscopic identification of the area of concern (arrow); C. The lesion was revealed to be a combined naevus, with a minor deep penetrating naevus component corresponding to the area of deeper pigmentation (arrow).
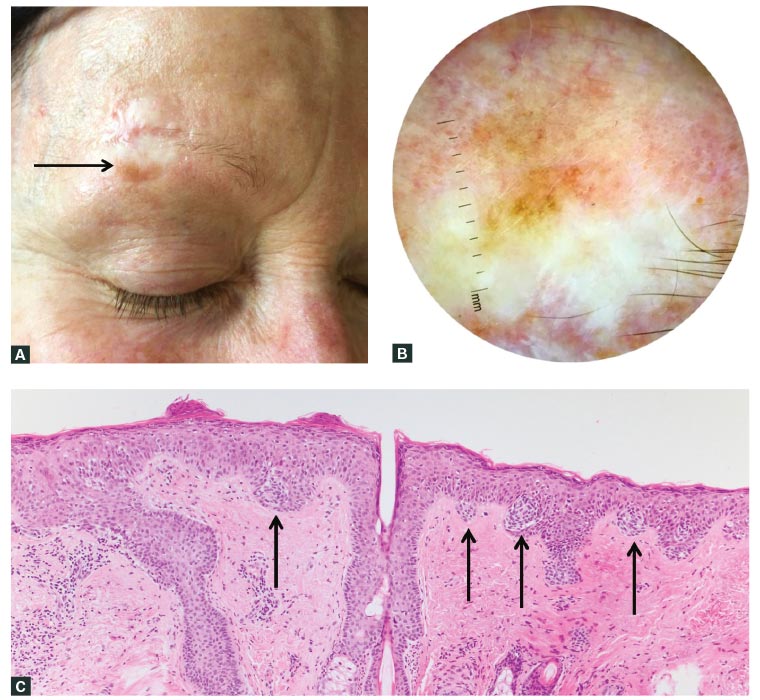
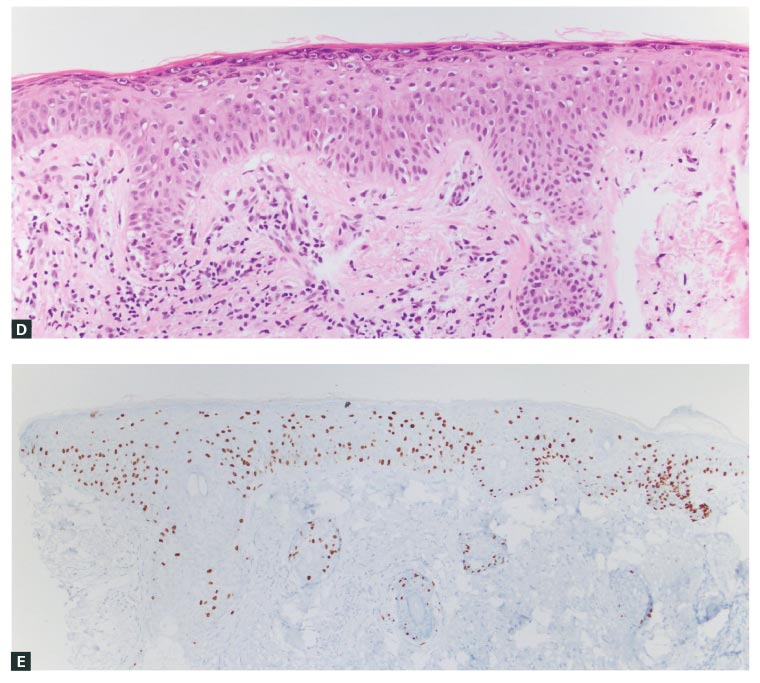
Figure 2. Resolving discrepancy between the clinical and pathological findings: New pigmentation adjacent to a surgical scar
A. A pigmented macule arose adjacent to a scar three years after surgery for lentigo maligna melanoma, as seen clinically; B. Dermoscopic view of the pigmented macule shown in A; C. The biopsy showed a discretely nested proliferation of melanocytes resembling a dysplastic naevus (arrows indicate nests of melanocytes). As a result of the discrepancy between the clinical and pathological findings, additional levels and SOX10 immunohistochemistry were performed; D. Areas of lentiginous growth with Pagetoid scatter were found on haematoxylin and eosin stains; E. Areas of lentiginous growth with Pagetoid scatter were found on SOX10 immunohistochemistry.
The final consensus diagnosis was that of a dysplastic naevus-like lentigo maligna.
Areas of particular concern in lesions
Any clinically or dermoscopically identified suspicious areas should be examined histopathologically because they may indicate melanoma. For example, a long-standing lesion with a recent change in colour or texture may suggest a melanoma developing within a pre-existing naevus. Such areas should be carefully described or marked for sectioning (eg with a suture or by superficially scoring the epidermis and superficial dermis around the area of concern, using a suitably-sized micro punch or other technique)21 to allow identification at the time of pathological processing of the specimen and assessing of the slides.22 Whatever method is used to identify the area of change, it should be clearly described on the pathology form or on an attached sheet. Clinical findings and/or the results of diagnostic imaging (eg dermoscopy or confocal microscopy) and/or a diagram should be included with the clinical request form if this information is likely to be useful for diagnosis. Such information can direct the pathologist to areas of particular clinical concern in the specimen, where deeper sections may be examined, or to improve clinicopathological correlation. Copies of photographic clinical or dermoscopic images provided with the specimen request form or sent via encrypted electronic email transfer can also be helpful when assessing clinically or dermoscopically heterogeneous lesions to direct the pathologist to areas of particular clinical concern. Occasionally, on request, the provision of microscopic photographs of the histopathology can be helpful for clinicians to correlate the histopathological features with the clinical scenario. Another way to resolve clinicopathologic discrepancies, diagnostic difficulties or management conundrums is for the pathologist and clinician to review both the histopathology and clinical and/or dermoscopic features together. Although infrequently required, such discussions can be of great value in properly elucidating difficult cases.
Discrepancy between clinical features and pathology report
If either the clinician or pathologist considers that there is a discrepancy between the clinical features and the pathological interpretation, discussion should be initiated with the other party about the case and an attempt made to determine the cause of the discrepancy. Excisional biopsy is strongly recommended when a small partial biopsy (usually a punch biopsy) of a possible melanoma is reported as a benign melanocytic lesion. Small punch biopsies are not recommended in the guidelines because they may not be representative and do not allow assessment of architecture, symmetry and focally suspicious changes; errors of interpretation may occur in as many as one in four melanomas sampled by such biopsies.9,23 When there is difficulty in resolving the reason(s) for any discrepancy, it may be appropriate to consider referring the case to another pathologist with special expertise in the interpretation of melanocytic tumours.23,24
Information that should accompany melanoma wide local excision specimens
When a diagnosis of melanoma is established, it usually requires a wide re-excision to ensure that the entire lesion is removed, primarily with the intention of reducing the risk of local recurrence. When submitting a wide re-excision specimen, it is important to communicate to the pathologist whether or not the melanoma was reported to be completely excised originally, the method of initial biopsy used (punch, shave, excision) and, if shave, whether the tumour reached the deep or peripheral margins of the shave specimen.
Conclusions
Clinical information is of critical importance for accurate pathological diagnosis of any tumour, but particularly for melanocytic skin tumours. Facilitation of accurate diagnosis and appropriate management of patients can be optimised by clinicians providing the pathologist with relevant clinical information that might influence the pathological diagnosis of melanocytic tumours.