Anaemia in pregnancy is a significant global health problem, with 38.2% of women worldwide affected,1 predominantly because of iron deficiency. Other causes include nutritional deficiencies, haemoglobinopathies, infectious and chronic diseases and, rarely, malignancy.2,3 Geographic variation occurs with greater prevalence in less developed countries,1 especially in households with low income.4 Australian prevalence rates are estimated at 25%,1 with elevated risk for Aboriginal and Torres Strait Islander women.5
Anaemia is defined by the World Health Organization as a haemoglobin (Hb) <110 g/L at any stage of pregnancy and <100 g/L postpartum.1 Physiological changes occur in the second trimester, increasing plasma volume alongside a smaller increase in red cell mass resulting in haemodilution – recognised as ‘physiological anaemia’. Therefore, a threshold of Hb <105 g/L in the second trimester is widely used throughout international guidelines in defining and directing management.2,3,6–8
The capacity to reduce rates within Australia depends on early diagnosis, risk detection and management. As part of preconception counselling or early antenatal care, it is important to consider known risk factors for developing anaemia in pregnancy, which include younger age (<18 years), multiparity, previous iron deficiency, shortened pregnancy interval, disadvantaged socioeconomic status, poor nutrition, non-white ethnic origin, haemoglobinopathy, chronic blood loss and parasitic disease.9–11 It is also vital to identify women at increased risk from the effects of anaemia, such as women at risk of haemorrhage at birth, who have bleeding disorders, are on anticoagulation therapy or who, for religious or cultural reasons, might decline blood products.
Iron deficiency anaemia
Iron deficiency anaemia (IDA) accounts for approximately 50% of cases worldwide.1 However, few studies have determined rates of IDA in an Australian pregnant population according to consistent definitions. A Tasmanian study reported prevalence of IDA at 18% among women in pregnancy using an Hb level of <115 g/L,12 and a New South Wales population study reported rates of low ferritin, defined as <12 ng/ml, as 19.6% in the first trimester.13 There is a pressing need to establish the true prevalence of IDA in pregnancy within Australia and identify subgroups of women, such as Aboriginal and Torres Strait Islander women, who are particularly vulnerable and for whom we can target treatment and preventive strategies.
Australian recommendations for dietary intake of iron for women in pregnancy averages out to 27 mg/day.14 Physiological demand for iron is three times greater during pregnancy, and a total of 1000–1200 mg iron is required overall. This physiological demand for iron increases from the second trimester and reaches its peak in the third trimester.3,6,7,15
Serum ferritin levels are used to assess iron stores in pregnancy. Once again there is variability in
levels to diagnose iron deficiency;
6,16 however, a serum ferritin level of <30 ng/mL is commonly used.
16 Ferritin should be evaluated alongside trends of red cell indices and clinical history.
3,6,7 This is particularly important within Australia’s mixed population in which underlying haemoglobinopathies are a potential concern (Figure 1).
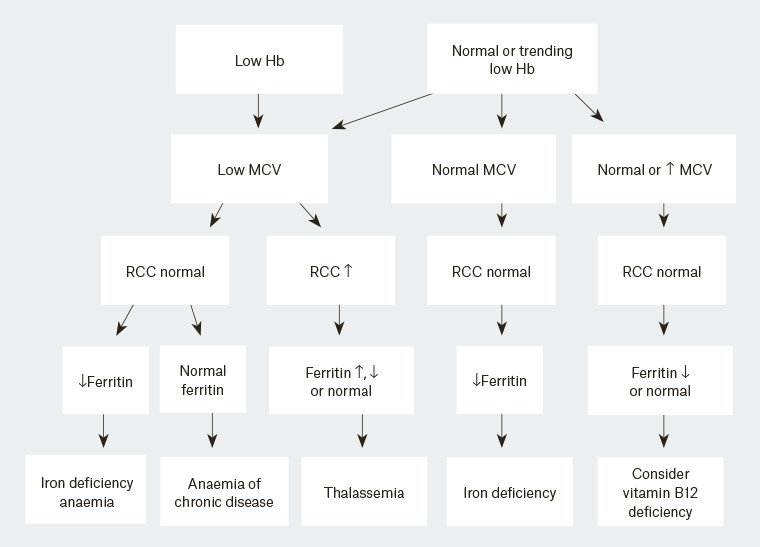
Figure 1. Interpretation of pathology results
Hb, haemoglobin; MCV, mean corpuscular volume; RCC, red cell counts
At present, routine administration of iron supplements in pregnancy is not recommended in Australia,17 the UK,3,6 New Zealand18 and the US19 because of concern regarding lack of evidence to support improved clinical outcomes on non-haematological parameters.20 Current practice in Australia recommends screening full blood examination at the first antenatal visit and at 28 weeks’ gestation, with further investigation and treatment as required on the detection of anaemia.17
Uncertainty remains around the importance of iron and physiological changes in pregnancy, and around the risk of not only too little but too much iron.6 Iron has a U-shaped nutrient health relationship, with functional impairment at one end of the curve and cytotoxicity at the other, with concerns regarding maternal haemoconcentration, pre-eclampsia and gestational diabetes with increased iron status.15,20 IDA, however, has been associated with poor gestational weight gain, fetal growth restriction, preterm delivery, increased risk of birth complications21 and depression in the mother.22 IDA in the mother can, for the newborn, also result in IDA23 and in behavioural and cognitive disorders,24 and has been associated with retinopathy of prematurity.25
Treatment recommendations of iron deficiency in pregnancy are outlined in Figure 2. Oral iron therapy remains the first-line treatment for IDA and iron deficiency26 (Table 1), with evidence supporting lower dose (20 mg/day) being as effective as high dose (80 mg/day).27 This is reassuring given that side effects (most commonly gastrointestinal [eg nausea, constipation]) with oral therapy are dose related. However, higher doses may need to be administered in IDA. Regardless, the response to therapy should be monitored, and, if inadequate and IDA occurs, intravenous (IV) iron administered in the second and third trimesters. Although recent studies have shown the use of IV iron in pregnancy is well tolerated, with both ferric carboxymaltose and iron polymaltose infusions,28 there remains a lack of evidence on maternal and neonatal outcomes.20,28 Once haemoglobin is normalised, replacement should continue for three months and/or until six weeks postpartum.5
| Table 1. Common oral iron formulations |
| Supplement name |
Iron content |
Elemental iron content |
| Ferro-Grad C |
Ferrous sulfate 325 mg |
105 mg |
| Ferro-Gradumet |
Ferrous sulfate 325 mg |
105 mg |
| Maltofer tablets |
Iron polymaltose 370 mg |
100 mg |
| Maltofer syrup |
Per 5 mL = Iron polymaltose 185 mg |
Per 5 mL = 50 mg |
| Ferro-F-Tab |
Ferrous fumarate 310 mg |
100 mg |
| Fefol delayed release iron |
Ferrous sulfate 270 mg |
87.4 mg |
| FGF |
Ferrous sulfate 270 mg |
80 mg |
| Ferro-Tab (PBS listed) |
Ferrous fumarate 200 mg |
65.7 mg |
| Ferro-Liquid |
Per 5 mL = Ferrous sulfate 150 mg |
Per 5 mL = 30 mg |
| Elivit |
Ferrous fumarate 183 mg |
60 mg |
| Elivit Women’s Multi |
* |
5 mg |
| Floradix |
Ferrous gluconate |
Per 10 mL = 7.5 mg |
| Spatone |
* |
25 mL sachet = 5 mg |
| *Denotes information not provided on products |
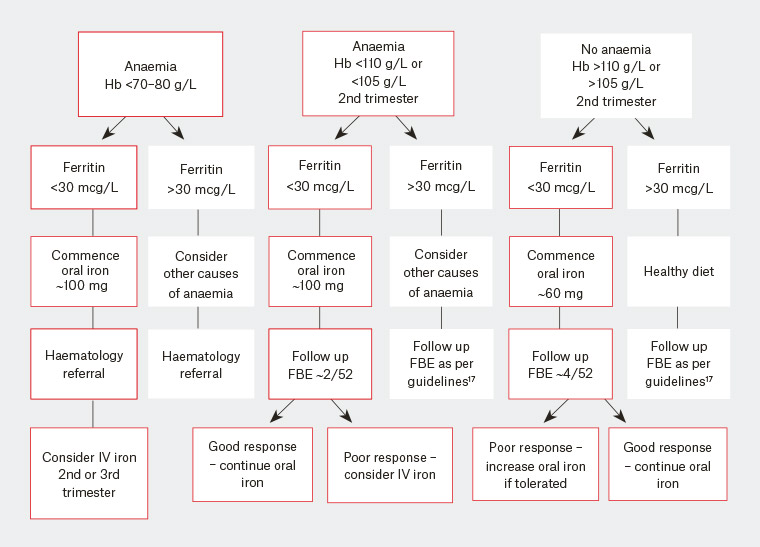
Figure 2. Management of iron deficiency
FBE, full blood examination; Hb, haemoglobin; IV, intravenous
Folate, B12 and micronutrient deficiencies
Historically, folate deficiency was the second most common cause of anaemia in pregnancy, but this is being overtaken by vitamin B12 deficiencies, particularly since folate supplementation in pregnancy is advised17 and with routine food fortification.2 Folic acid supplementation of 0.4 mg/day in the first 12 weeks and 2.6 micrograms/day for B12 throughout the pregnancy is recommended.17
Routinely, assessment of B12 should occur in all women with vegetarian and vegan diets; malabsorption disorders such as Crohn’s disease and coeliac disease; autoimmune diseases; medication use (eg metformin); and, increasingly, in those who have had bariatric surgery, particularly gastric sleeve surgery. Measurement of total B12 is first line but has limitations in diagnosis. Active B12 is currently triggered on pathology for those with low levels and is a more sensitive marker. Folate and vitamin B12 are essential for DNA synthesis and nuclear maturation. Deficiencies are associated with higher risk of neural tube defects, and have possible links with infertility, recurrent spontaneous abortion and preterm birth.21
Correction of B12 deficiency is important because deficiency may also occur in the newborn and has been associated with neurological symptoms in infants exclusively breast-fed.17 There remains a lack of evidence guiding optimum treatment of B12 deficiency in pregnancy in regard to oral versus intramuscular replacement. Decision on administration route must be based on patient preference, reason for B12 deficiency and the possibility of poor oral absorption.
Other micronutrient deficiencies, such as in vitamins A and C, zinc and copper, can occur but are difficult to measure, with the prevalence as a cause for anaemia in women who are pregnant unknown. Multiple nutritional deficits often coexist in underprivileged communities. Supplementation has been shown to improve maternal anaemia in specific at-risk populations2 and should be encouraged throughout the pregnancy in these populations.
Haemoglobinopathies
Haemoglobinopathies can be divided into thalassaemias and haemoglobin variants such as sickle cell disease (SCD). Thalassemia is an inherited disorder associated with impaired synthesis of one or more of the globin chains, with alpha and beta thalassemia being most common. The clinical significance is complex and variable, and haematological opinion should be sought (Table 2).
| Table 2. Summary of significant haemoglobinopathies that may affect the woman and fetus |
| Haemoglobin disorder |
Maternal genotypes that may affect the woman and the fetus (depending upon partner study results) |
| Alpha thalassaemia |
αα/α-, αα/--, α-/α- (carrier or minor trait)
α-/-- (haemoglobin H disease)
--/-- (Barts hydrops generally resulting in death in utero) |
| Beta thalassaemia |
β/β0, β/β+ (carrier or minor trait)
β+/β+, β0/β+ (Beta thalassemia intermedia)
β0/β0 (Beta thalassemia major) |
| Haemoglobin S |
AS (sickle cell trait)
SS, SC, S/β thal., SD, S/O-Arab (sickle cell disease) |
| Haemoglobin E |
AE (carrier)
E/β thal. (ranges from E/β thal. intermedia to major) |
There is no national screening program for haemoglobinopathies within Australia and as a result there is variability in testing, but identifying vulnerable populations is important29 (Table 3). Haemoglobinopathy studies should be performed at preconception or in early pregnancy to identify partners who require screening, fetuses at risk of significant disease, and to offer informed choice and counselling, prenatal diagnosis and planning for infant care.6,29
| Table 3. Risk groups for haemoglobinopathies |
| Clinically significant haemoglobin disorder |
Area of prevalence/family origin |
| Alpha thalassaemia |
Southeast Asia, India, Middle East, Africa and Mediterranean |
| Beta thalassaemia |
Southeast Asia, Indian subcontinent, Middle East, Africa and Mediterranean |
| Haemoglobin S |
Africa including North Africans, African American, African Caribbean, British African or any other African ethnicity |
Iron deficiency can coexist with thalassemia; conversely, the more severe thalassaemic syndromes are associated with iron loading.30 Women in pregnancy with an unknown haemoglobinopathy status should be commenced on a trial of oral iron while screening is being undertaken. However, screening should be undertaken prior to the administration of IV iron, as elevated ferritin levels may affect results.6
SCD is a group of inherited autosomal recessive disorders that affect haemoglobin structure31 and, with an increase in migration, is being seen more frequently within Australia. Folic acid 5 mg daily should be given both preconceptually and throughout pregnancy in women with SCD.31 HbS combined with normal haemoglobin (A) is called ‘sickle cell trait’ and is frequently asymptomatic.
Infections
Although not as common, maternal helminthic infection with Ascaris lumbricoides, Trichuris trichuria or hookworms carries increased risk of anaemia during pregnancy.32 Treatment with antihelminth therapy is considered safe in pregnancy, but iron replacement is still often required to correct iron deficiency.
Malaria, despite being rarely seen in Australia, should be considered in anaemia, particularly as many women in pregnancy still travel to risk areas and antimalarial prophylaxis is limited in this group of travellers. Severe anaemia, as acute haemolytic anaemia, has been reported with malaria infection.2
Human immunodeficiency virus (HIV) is an independent risk factor for anaemia in pregnancy and is thought to be caused through chronic inflammation.2
Summary
General practice provides an important opportunity for early identification of anaemia in women contemplating, or in the first trimester of, pregnancy. Although routine screening of haemoglobin occurs in Australia, targeting high-risk groups can enable early management and improve outcomes. Investigations determine the underlying aetiology and guide management. Current evidence recommends oral iron therapy first line for IDA and iron deficiency, with appropriate follow-up. Haematological referral should be sought in complex cases.