General practitioners (GPs) often care for women and couples who are preparing to start or continue their family. GPs are able to provide preconception planning to optimise the health and wellbeing of the couple and their child.1 Multiple tests are offered to identify potential risks to a couple’s future child, including testing for maternal infections and nutrient levels. One important risk for which GPs do not routinely offer testing to couples is the risk of autosomal recessive and X-linked genetic conditions that cause severe disabilities and significantly affect the quality of life for the child and their family. Such testing is available commercially, including as direct-to-consumer testing.1 In the 2018 federal budget, Mackenzie’s Mission, a $20 million Medical Research Future Fund research project investigating carrier screening, was announced; the aim of Mackenzie’s Mission is that carrier screening for inherited disorders will become freely available to all Australians.
There are >5000 conditions for which the genetic basis has been elucidated, and this number is increasing weekly.2 Many of these are inherited in an autosomal recessive or X-linked fashion. If both members of a couple are carriers (heterozygous) for an autosomal recessive condition, they have a one in four chance with each pregnancy to have a child affected by that condition (Figure 1a). Examples of autosomal recessive conditions include cystic fibrosis (CF), spinal muscular atrophy (SMA) and Tay-Sachs disease (TSD). If a woman is a carrier of an X-linked condition, she has a one in four chance of having a son with that condition in each pregnancy (Figure 1b). Examples of X-linked conditions include fragile X syndrome (FXS), haemophilia A and B, and Duchenne muscular dystrophy. Approximately 1–2% of non-consanguineous couples have a one in four chance of having a child with an autosomal recessive or X-linked recessive condition.3 The risk is considerably higher for consanguineous couples.4
The aim of genetic carrier screening is ‘to facilitate informed reproductive decision making by identifying those couples at risk of having an affected child’.5 Carriers can be identified by genetic testing, and at-risk individuals and couples have the option to take steps to avoid having affected children.
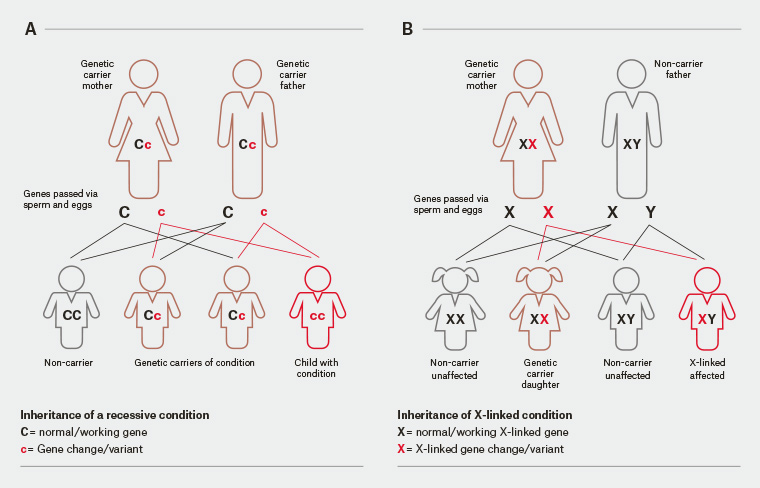
Figure 1. Inheritance patterns: A. autosomal recessive and B. X-linked recessive inheritance. For some X-linked conditions (such as fragile X syndrome), females who carry a mutation may display some features of the condition.
History
In the 1970s, prior to the first gene being cloned, it became possible to screen for carrier status for two conditions: haemoglobinopathies such as beta-thalassaemia and sickle cell disease,6 and TSD7. Carrier screening for these conditions was possible because carriers have blood parameters that differ from non-carriers (ie abnormalities on haemoglobin electrophoresis for haemoglobinopathies and lower levels of the enzyme hexosaminidase A for TSD). Screening programs for these disorders were therefore established in high-risk communities.
Once mutations in genes were identified as the cause of genetic disorders, screening for an ever-increasing number of disorders became possible. The first disorder for which widespread screening became available by genetic testing was CF.8 This was initially by targeted testing for common CFTR mutations. In recent years it has become possible to ascertain the DNA sequence of many genes at once (massively parallel sequencing, also called next generation sequencing) and therefore identify carriers not only of common mutations, but also of rare mutations. The advent of massively parallel sequencing has meant that it does not cost much more to screen for hundreds or thousands of conditions than it does to screen for a few. This form of screening is called expanded carrier screening and is now widely available.5 There are a number of companies in Australia and internationally offering expanded carrier screening for over 100 genetic conditions.3
Reproductive carrier screening has been offered in a number of settings. High school screening has been offered in particular to the Ashkenazi Jewish community.9,10 The Ashkenazi Jewish high school screening program has been ceased in Melbourne because of the likelihood that current high school students will have access to more extensive screening by the time they are planning their families. Screening is more commonly offered by health professionals to women and couples planning, or in the early stages
of, pregnancy.1,11
How does screening work?
Table 1 outlines key issues in considering carrier screening. In theory, it is possible to screen for any autosomal or X-linked condition for which the genetic basis is known. In practice, screening is generally offered for conditions where the impact on the affected individual is considered significant and the condition is reasonably common.5
| Table 1. Considerations for practitioners offering genetic carrier screening |
| Who? |
Carrier screening is relevant to everyone
- Most carriers of recessive conditions have no family history of the condition; therefore, carrier screening should be offered regardless of presence or absence of family history.
- If there is a family history of a particular genetic condition, it is important to convey family history information to the testing laboratory. This will ensure the appropriate test is performed and will reduce the chance of a false negative result.
|
| When? |
Carrier screening is most relevant pre-pregnancy and in early pregnancy
- Preconception carrier screening is ideal as the widest range of reproductive options are available at this time, and it allows couples at increased risk time to consider these options.
- If screening is offered in early pregnancy, a couple screening approach may be preferable as results will be received in a timelier manner.
|
| What? |
Guidelines recommend offering carrier screening for:
- haemoglobinopathies (initially via full blood examination and haemoglobin electrophoresis)
- cystic fibrosis
- fragile X syndrome
- spinal muscular atrophy.
Expanded carrier screening panels offering screening for a large number of conditions are also available. |
| How? |
Women/couples should be given the opportunity to make an informed decision about carrier screening
- Genetic testing laboratories usually provide information brochures and have web information about carrier screening for healthcare providers to give to their patients.
- Women/couples may appreciate the opportunity to discuss the pros and cons of screening with their healthcare provider.
- For those requesting more detailed information, the opportunity to speak to an expert may be offered through the genetic testing laboratory or a local clinical genetics service.
Carrier screening is a simple blood or saliva test
- Complete a pathology request form requesting carrier screening for the patient.
- Testing can be arranged by ordering a test kit directly from the genetic testing laboratory or by the patient having their sample taken at a local pathology collection centre.
|
| Where? |
Testing for genetic carrier status is ideally performed by specialist genetic testing laboratories
- Offering a genetic carrier screen provided by a service that includes genetic counselling may simplify the process for general practitioners. These services provide easy access to expert interpretation, information and support as required. They may also offer comprehensive genetic counselling for couples at increased risk.
- For information about carrier screening options in your state, contact your local clinical genetics service.
|
Some mutation types cannot be identified by massively parallel sequencing, and therefore require specific, targeted assays to detect carriers. Two important examples are nucleotide repeat disorders and disorders due to copy number variants, the most common of which are FXS and SMA, respectively.
12 FXS is mainly caused by an increased number of CGG repeat units upstream of the
FMR1 gene. The majority of carriers of SMA are missing part or all of one copy of
SMN1.
The two main ways in which screening is undertaken are sequential and couple screening. In sequential screening, one partner is tested first, and the second is only tested if the first is a carrier of an autosomal recessive condition. In general, the woman is tested first, as it is the woman’s result that is important for X-linked conditions such as FXS. In couple screening, both members of the couple are tested simultaneously. They are given one of two results: an increased risk of having a child with a genetic condition if both are carriers of the same autosomal recessive condition or the woman is a carrier of an X-linked condition, or a low risk if the couple are not carriers of the same autosomal recessive condition and the woman is not a carrier of an X-linked condition.5 Sequential screening is generally cheaper for the couple if they are paying, since the majority of couples will only require one person to be tested if the person tested is not found to be a carrier of any of the conditions for which screening is done. Sequential screening also enables carrier testing of close relatives (cascade testing) who are at increased risk of being a carrier of a genetic condition because of a family member being identified as a carrier of a genetic condition.
The chance of being identified as a carrier increases considerably as the number of conditions screened increases. This has significant implications for genetic counselling resources. For example, if only CF screening is offered, approximately one in every 30 tested individuals in Australia require genetic counselling and testing of their partner.12,13 Once a large, expanded reproductive panel of disease mutations is used, then up to 50% of individuals will be carriers of at least one condition.3 Couple screening therefore becomes more practical.
The advantages of couple screening are twofold. First, far fewer people require genetic counselling, as most couples do not carry the same condition, and therefore they receive a combined ‘low risk’ result. Second, a definitive result is available in a shorter time frame, which is advantageous when screening is done once the woman is already pregnant. The lost opportunity for cascade family carrier testing is negated if couple screening is offered widely in a community.
A common misconception is that screening is not necessary if a couple have no known family history of a genetic disorder. Almost 90% of carriers of CF, SMA and FXS have no known family history of the condition.12 For conditions less common than these, it is even less likely that a family history will identify at-risk couples.
It is critical that screening is offered as an option rather than as a ‘routine test’ done before or early in pregnancy, and that every effort is made to facilitate an informed choice by couples about whether or not to be screened. Individuals and couples contemplating screening should be made aware that having screening can lead to difficult decisions about current and future reproductive options. Couples who choose not to have screening and who have a child with an autosomal recessive or X-linked recessive condition must be fully supported.
What are the options for couples found to be at increased risk of having a child with a condition for which screening is offered?
For couples who are found to be at increased risk, there are a number of options to avoid the birth of an affected child. These include not having children; adoption; using donor egg, sperm or embryo; testing an established pregnancy by chorionic villus sampling (CVS) or amniocentesis; or testing embryos produced by in vitro fertilisation (IVF) by preimplantation genetic diagnosis (PGD).14 Many will choose one of the latter two options. All options are available to couples who learn of their increased risk carrier status prior to pregnancy; when the woman is already pregnant, the only available option is testing an established pregnancy by CVS or amniocentesis, reinforcing the preference for carrier screening taking place pre-pregnancy.
What do Australasian peak body guidelines recommend?
The Royal Australian and New Zealand College of Obstetricians and Gynaecologists (RANZCOG) guideline states:
Carrier screening for the more common ‘monogenic’ genetic conditions (eg CF, SMA, FXS, haemoglobinopathies) is available in Australia and in New Zealand (in the private sector). Information on carrier screening for the more common genetic conditions that affect children (eg CF, SMA, FXS) should be offered to all women planning a pregnancy or in the first trimester of pregnancy. Women wanting more information about carrier screening should be given the opportunity to have a more detailed discussion about carrier screening with an informed clinician. The benefits and limitations of testing, and any associated costs should be discussed.15
Notably, RANZCOG has changed its guideline from ‘carrier screening may be offered’ in the previous version of the guideline to ‘carrier screening should be offered’ in the latest version. The Royal Australian College of General Practitioners (RACGP) released the information resource Genomics in general practice, in 2018.16 It states:
All women or couples planning a pregnancy, or who are already pregnant, should have a comprehensive family history recorded. Women or couples who are known carriers of a genetic condition or have a relevant family history should be made aware of the availability of carrier screening and offered referral to specialist services (ie genetics or obstetrics). Carrier screening for common recessive genetic conditions (eg CF) may be offered to low-risk women or couples (ie regardless of family history and ethnicity). The decision to have screening is a personal choice to be made by the individual or couple.
What is the effect of being found to be at increased risk of having a child with a condition for which screening is offered?
A study of the first 12,000 individuals screened for carrier status for CF, SMA and FXS in Victoria found that one in 20 individuals was a carrier of at least one of the three conditions.12 Seventy-seven per cent of tests were ordered by an obstetrician or fertility specialist and 19% by a GP. The majority of women were pregnant at the time of screening. There were 14 couples identified as being at increased risk for CF, one for SMA and 35 for FXS. In this program, couples at increased risk are offered consultation with a specialist in the condition (respiratory paediatrician for CF and neurologist for SMA). Of the 32 women pregnant at the time of being identified as being at increased risk, 26 had prenatal diagnosis. There were seven affected pregnancies, six of which were terminated.12
Research has shown that people are often anxious when told they are carriers, but this anxiety dissipates when their partner is shown not to be a carrier.5 Most carriers do not regret being tested.17 Most individuals and couples identified as being at increased risk of having a child with one of the conditions screened, while appropriately distressed about this result, undertake steps to avoid having a child with the condition and do not regret having screening.12,13
Mackenzie’s Mission
In the 2018 federal budget, $500 million was announced to fund the Australian Genomics Health Futures Mission from the Medical Research Future Fund. The first project in this initiative is Mackenzie’s Mission, for which $20 million has been earmarked. Mackenzie’s Mission is named for Mackenzie Casella, a child who died at seven months from SMA in 2017 and whose parents asked why they were not aware that they are both carriers of SMA and therefore had a one in four chance of having a child with this condition. Mackenzie’s Mission aims to screen 10,000 couples for carrier status for approximately 500 autosomal and X-linked genes. Couples will be recruited from all Australian states and territories through specific general practices, public obstetric hospitals, private obstetricians, IVF practices and clinical genetics units. Mackenzie’s Mission will work with a range of metropolitan and rural practices, and these practices will receive education and support prior to and during the project. Research outcomes will include uptake of screening, frequency of carrier status and of increased risk couples, reproductive decisions of those identified at increased risk, health economic outcomes, ethics research, health implementation research and psychosocial outcomes. It is expected that screening in this project will commence in late 2019.
Key points
GPs are crucial to any future carrier screening program. The best time for screening to take place is prior to pregnancy, so that all reproductive options are available. GPs are the health professionals most likely to see individuals and couples prior to pregnancy. As couples planning, or in the early stages of, pregnancy are exposed to advertising from companies offering carrier screening and/or are informed about screening by families and friends,18 GPs will increasingly be asked about what screening is available and how it can be accessed. As many GPs are not currently familiar with carrier screening, it is important for them to upskill to be comfortable offering this testing.19 Further research is needed to identify approaches that enable GPs to offer screening in a way that provides sufficient information and support to their patients while minimising the impact on GP time and resources.19 Additionally, it is important that the appropriate resources are available to GPs for education about carrier screening as well as pathways for referral for comprehensive clinical support when it is needed.19 We are researching the best and most efficient ways of offering carrier screening services and strategies for upskilling GPs, and will continue this research in Mackenzie’s Mission for both metropolitan and rural GP practices. If Mackenzie’s Mission is successful, it will hopefully lead to carrier screening being freely available to all Australians in the future as standard of care. GPs and obstetricians, in partnership with genetic services and pathology laboratories, will be at the forefront of this testing.