Case
A woman aged 22 years presented to a headspace community health nurse with symptoms of anxiety, amotivation and low mood since mid–high school. She had been using marijuana regularly and had recently deferred her university course. A psychologist whom she had previously seen had diagnosed anxiety and body dysmorphia. The patient was referred to a headspace counsellor. Incidentally, marked hirsutism was noted by the nurse and an appointment with a headspace general practitioner (GP) was scheduled. The patient had engaged well with the counsellor by the time of her GP appointment.
The GP undertook history-taking and examination. The patient’s menarche had occurred at 14 years, and she had a regular 28-day cycle. She had mild facial acne at puberty, but no acne on her chest or back. The patient had become increasingly hirsute since her mid-teens but noted that none of her three sisters are hirsute. She was not using anabolic steroids. There is no diabetes or heart disease in her immediate family. She was health conscious and had been vegan for 12 months, with weight loss of 10 kg over the past 12 months, which she attributed to dietary changes. She had experienced tiredness for several years, but no abdominal pain, gastrointestinal or genitourinary symptoms. The patient’s weight was 60.1 kg, body mass index was 21.3 kg/m2, blood pressure (BP) was 121/83 mmHg and pulse was 84 beats per minute. Her abdomen was normal and breasts were small. When examining for signs of virilisation, the GP noted marked coarse, dark, male distribution hair pattern on face, abdomen, lower back, inner thighs, sternum and breasts, with androgenic alopecia, laryngeal enlargement with deepened voice and muscle hypertrophy. Genital examination was declined. The patient was clinically euthyroid and not cushingoid.
Question 1
What are the differential diagnoses?
Answer 1
The differential diagnoses are:
- androgen-secreting adrenal or ovarian tumour with significant risk of malignancy, until proven otherwise
- late-onset (non-classic) congenital adrenal hyperplasia (CAH)
- polycystic ovary syndrome (PCOS)
- ovarian hyperthecosis
- syndromes of severe insulin resistance
- exogenous androgen use (including accidental transfer of testosterone gel).
Question 2
What tests should be ordered?
Answer 2
In addition to trans-abdominal and transvaginal pelvic ultrasonography, tests should be ordered to investigate:
- follicle-stimulating hormone (FSH)
- luteinising hormone (LH)
- oestradiol
- prolactin
- testosterone (female)
- sex hormone binding globulin
- calculated free testosterone
- dehydroepiandrosterone sulfate (DHEAS)
- progesterone
- 17-hydroxyprogesterone
- androstenedione
- ·adrenocorticotropic hormone
- 24-hour urine-free cortisol
- cortisol
- dexamethasone suppression test (although not cushingoid, important to exclude Cushing’s disease because most androgen-secreting adrenal tumours also secrete cortisol)
- thyroid-stimulating hormone
- electrolytes, urine, creatinine
- glomerular filtration rate
- liver function
- full blood evaluation
- erythrocyte sedimentation rate
- C-reactive protein
- ferritin (vegan, tiredness).
The patient’s results can be seen in Table 1.
The patient’s ultrasound results showed:
- left ovary enlarged (22 cc); both ovaries showed >25 follicles
- a well-defined 40 mm vascular lesion adjacent to the right kidney, isoechoic to the renal parenchyma. Unable to determine if renal or adrenal in origin. Contrast enhanced adrenal computed tomography (CT) recommended.
| Table 1. Results |
| Investigation |
Result |
| Follicle-stimulating hormone |
12.7 IU/L |
| Luteinising hormone |
38 IU/L |
| Prolactin |
210 mIU/L (reference range 59–619 mIU/L) |
| Testosterone (female) |
6.2 nmol/L (reference range 0.2–1.8 nmol/L) |
| Calculated free testosterone |
114 pmol/L (reference range 13–39 pmol/L) |
| Dehydroepiandrosterone sulfate |
47.1 µmol/L (reference range 1.0–11.7 µmol/L) |
| Sex hormone binding globulin |
26 nmol/L (reference range 28–146 nmol/L) |
| Androstenedione |
18.7 nmol/L (reference range 1.0–12.9 nmol/L) |
| Oestradiol |
199 pmol/L |
| Progesterone |
13 nmol/L |
| 17-hydroxyprogesterone, early am |
6.1 nmol/L (reference range <8.0 nmol/L) |
| Adrenocorticotropic hormone |
46 pg/ml (reference range <46 pg/ml) |
| Cortisol am |
484 nmol/L (reference range 119–618 nmol/L) |
| 24-hour urine-free cortisol |
Normal |
| Dexamethasone suppression test |
Normal |
| Thyroid-stimulating hormone |
0.580 mU/L (reference range 0.30–4.00 mU/L) |
| Electrolytes, urine, creatinine; estimated glomerular filtration rate |
Normal |
| Liver function tests |
Normal |
| Full blood evaluation |
Normal |
| Erythrocyte sedimentation rate |
2 mm/hr |
| C-reactive protein |
<2.9 mg/L |
| Ferritin |
Normal |
Question 3
What do these results suggest?
Answer 3
The results satisfy criteria for PCOS, with two of three diagnostic criteria – polycystic ovaries and hirsutism – having been met (oligomenorrhoea is the third criterion). However, the patient was virilised, which does not occur in primary PCOS; this means she has PCOS secondary to virilisation.1 Polycystic ovaries occur in many of the causes of virilisation, including non-classic CAH.
Non-suppressed FSH and LH suggest no exogenous androgen.
High testosterone (>5 nmol/L), particularly DHEAS, is consistent with an androgen-secreting adrenal neoplasm. Absence of cushingoid phenotype, together with normal overnight dexamethasone suppression test and 24-hour urine cortisol, excluded excess glucocorticoid secretion. Normal BP and plasma potassium are inconsistent with excess aldosterone secretion.
Therefore, this is a pure androgen secreting adrenal tumour (PASAT), which is rare. In one series, 21 out of 430 (4.9%) of primary adrenocortical tumours were PASAT, of which 47.6% were malignant.2
Case continued
Results were discussed with the patient, and it was decided that further imaging would be arranged by an endocrinologist.
Contrast-enhanced CT of abdomen and pelvis showed a 40 mm right adrenal mass with characteristics suggestive of adenoma, but unable to exclude carcinoma (Figure 1). Magnetic resonance imaging was likewise inconclusive.
The patient was referred to a surgeon and underwent successful laparoscopic right adrenalectomy. Following surgery, androgens reduced dramatically. Three weeks post-surgery, testosterone was 2 nmol/L, DHEAS was 11.8 µmol/L and androstenedione was 14.1 nmol/L.
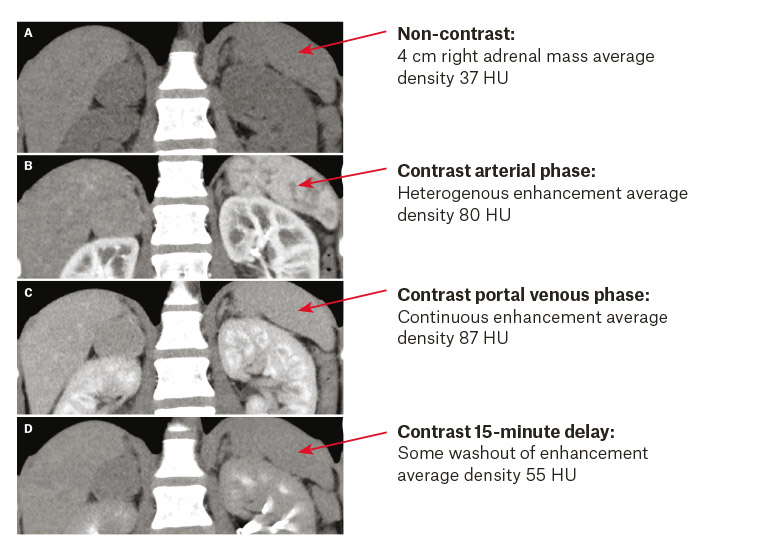
Figure 1. Adrenal computed tomography scan before contrast (A) and after contrast (B–D)
Histology showed a 51 mm ‘oncocytic adrenocortical tumour with uncertain malignant potential’. Second opinion on histology concurred.
No adjunct treatment was deemed necessary and, due to uncertain long-term malignant potential, specialist monitoring was advised.
Eight months post-surgery, testosterone of 1.7 nmol/L (reference range 0.4–2.1 nmol/L) and androstenedione of 8.9 nmol/L (reference range 1–12.9 nmol/L) were both normal. DHEAS was mildly elevated at 14.6 µmol/L (reference range 1.0–11.7 µmol/L).
Physical symptoms of virilisation had decreased, anxiety subsided, drug use ceased and the patient had successfully returned to her studies.
Key points
- Until proven otherwise, a female with secondary virilisation has an ovarian or adrenal tumour, which is quite likely malignant. Timely investigation is essential.
- PCOS can be secondary. Extreme androgenisation is inconsistent with primary PCOS. In PCOS cases with hirsutism or andogenic alopecia, serum testosterone >5 nmol/L requires exclusion of virilising tumour or enzyme deficiency.3
- Emotional wellbeing is associated with physical health, and visa versa. While this unusual case demonstrates the importance of easy access to primary care, GPs often have a role to play in the context of youth mental health. Having primary care onsite allowing easy in-service referral is particularly important at services such as headspace.