Melioidosis is a disease of humans (and animals) resulting from infection with the Gram-negative bacterium Burkholderia pseudomallei. Initially described by Whitmore and Krishnaswami in 1912 following reports of severe pneumonia and septicaemia in Burma,1 the first human case of melioidosis in Australia was described in 1950, in a patient with diabetes who died from septicaemic melioidosis in Townsville.1 The majority of Australian cases are reported in the Northern Territory (NT), North Queensland and the Kimberley region of Western Australia; although sporadic clusters and cases are acquired outside endemic regions in Australia, including southwest Western Australia (WA), Alice Springs and the Brisbane River Valley in Queensland.1
Microbiology
B. pseudomallei are small, Gram-negative, oxidase-positive, motile, aerobic bacilli that reside in soil, water and plants in endemic regions.2,3 B. pseudomallei closely resemble members of the genus Pseudomonas and were actually called Pseudomonas pseudomallei, before being separated into a new genus in 1992.1 Recent phylogenomic analyses using whole-genome sequencing, in addition to sophisticated phylogeographic techniques, show that ancestral strains of B. pseudomallei first arose in Australia, and then spread throughout Asia, with subsequent evolution and global dissemination.4 B. pseudomallei are hardy environmental saprophytes that resist temperature extremes, acidic and alkaline conditions, disinfectants and antiseptic solutions.1–3 As facultative intracellular pathogens, B. pseudomallei employ various mechanisms to facilitate survival within host innate immune system cells, enabling cell–cell bacterial spread and biofilm production.2,3
Epidemiology and risk factors
Documented peak rates of melioidosis occur in the NT1 and in North-eastern Thailand;5 however, infection is likely significantly under-diagnosed and under-reported throughout South East Asia, the Indian sub-continent, Sri Lanka, China and Papua New Guinea.1–3 There are increasing numbers of reports of sporadic cases from Central and South America, Africa, the Middle East, the Caribbean, the Pacific (eg New Caledonia) and Indian Oceans (eg Mauritius).2–3 The estimated annual global disease burden of melioidosis is approximately 165,000 cases, including 89,000 deaths.4
Human melioidosis occurs in people of all ages, although peak disease incidence occurs in adults aged between 40 and 60 years.2–3 Melioidosis incidence and disease severity is determined by host, environmental and strain-specific virulence factors. Retrospective reviews of Australian and Thai cohorts6–8 have identified many host risk factors for melioidosis including diabetes mellitus, heavy alcohol consumption (including binge drinking) and chronic kidney and lung disease. Individuals with increased exposure to soil and water in endemic areas are at highest risk of infection, including Aboriginal and Torres Strait Islander peoples, agricultural workers, construction workers and ecotourists.3,5 Strong correlations exist between melioidosis cases and mean monthly rainfalls (Figure 1),9 with epidemiologic studies demonstrating that 81% of Australian cases occur during the wet season.9 The predominant mode of transmission is percutaneous inoculation after exposure to wet-season soils or water.1–3 Acquisition by aerosols or aspiration events (eg near drowning) often follows heavy rainfall and severe storms and is independently associated with shorter incubation periods with higher risks of pneumonic presentation, septic shock and death;5,6,9 this probably reflects a shift towards transmission via inhalation, and possibly higher inoculating doses.6,9 Ingestion is clinically relevant in some endemic areas, particularly from unchlorinated potable water.1 Other modes of spread – including vertical transmission, zoonotic transmission, transmission via breast milk from mothers with mastitis and sexual intercourse – are rare, but are described.1 Nosocomial outbreaks are rarely documented.1–3 Details of epidemiology and risk factors are outlined in Table 1.
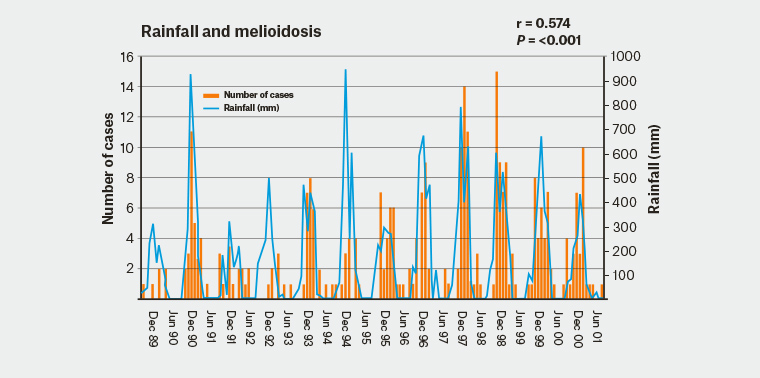
Figure 1. Monthly rainfall and number of melioidosis cases during the 12-year study period in the Northern Territory
Adapted from Currie BJ, Jacups SP. Intensity of rainfall and severity of melioidosis, Australia. Emerg Infect Dis 2003;9(12):1538–42.
| Table 1. Summary of clinical manifestations of melioidosis* |
| Asymptomatic seroconversion |
Most common, as suggested by seroprevalence studies in endemic regions1 |
| Many risk factors are described for infection1,† |
| Acute infection (85%) |
Mean incubation period approximately 9 days (range: 1–21 days), but presents much earlier following inhalational/aspiration events.1,9,‡ Wet season: October to April. |
| Symptom duration <2 months.1 Most develop acute melioidosis, with 50% bacteraemic on presentation, and approximately 20% develop septic shock5 |
| Pulmonary infection |
Adult presentations 51% (Figure 2); paediatric presentations 20%5,8 |
| Variable radiologic appearances: minimal infiltrates/cavitation/diffuse parenchymal disease8 |
| Cutaneous infection |
Adult presentations 13%; paediatric presentations 60%5,10 |
| Single lesions are typically at site of inoculation1 |
| Pyogenic infection |
Parenchymal visceral abscesses are common including spleen, liver, kidneys5 |
| Prostatic abscess (18%;1 Figure 3)§ |
Parotitis is rare in Australia1,ǁ |
| Central nervous system infection |
Intracerebral abscesses: thought to be secondary to bacteraemic spread1,2 |
| Encephalomyelitis#: typically produces brainstem signs11 (refer to case report) |
| Bone/joint infection |
Seen in 4% of cases, due to direct extension or through haematogenous spread5 |
| Other (rare) |
Mycotic aneurysms, pericarditis, mediastinal masses, thyroid and scrotal abscesses1,5 |
| Chronic infection (11%) |
Defined as symptom duration >2 months1 |
| Much better prognosis than acute melioidosis5 |
| Pulmonary infection |
Fevers, weight loss and productive cough; often mimics pulmonary tuberculosis5 |
| Predominantly upper lobe infiltrates on chest radiography5 |
| Cutaneous infection |
Non-healing cutaneous lesion, often unresponsive to multiple courses of antibiotics5 |
| Reactivation of latent disease (4%) |
Typically pulmonary reactivation disease5 |
| Latent periods of 14–24 years (possibly up to 62 years)1,5 |
| Risk factors |
Concurrent infections: influenza, bacterial sepsis1 |
| Traditional risk factors for disease are diabetes mellitus, hazardous alcohol intake, chronic renal disease and/or urolithiasis, and chronic lung disease1 |
*Clinical manifestations are subject to ascertainment bias and other forms of biases inherent to the source of individual case series
†In addition to traditional risk factors, putative risk factors for infection include malignancy, steroid and other immunosuppressive therapy, rheumatic heart disease and/or congestive cardiac failure, thalassaemia, iron overload, kava consumption, chronic granulomatous disease, pulmonary haemosiderosis and tuberculosis6–8
‡Incubation periods as short as <24 hours have rarely been reported1–3
§Prostatic abscess is most commonly detected in Australia, but appears rare in Thailand1–3
ǁSuppurative parotitis is a common presentation of melioidosis in children in Thailand and Cambodia, causing up to 40% of cases in Thai children.1 Parotitis is far less common in Australia.1
#Encephalomyelitis comprises a broad clinical spectrum, but clinically it is primarily a brainstem encephalitis. Prominent clinical features include cranial neuropathy (particularly VI, VII nerves), unilateral upper motor neuron limb weakness, cerebellar signs, bulbar palsy or flaccid paraparesis.1 Most patients have a normal or near-normal consciousness at presentation. Encephalomyelitis is rarely reported outside of Australia.1 |
Clinical features
The clinical features of melioidosis mimic many other diseases (eg sepsis/septic shock, community-acquired pneumonia [CAP] and tuberculosis), resulting in frequent misdiagnosis of the condition.5 Consequently, melioidosis is known as the ‘great mimicker’.1 The mean incubation period is nine days (1–21 days) but symptoms can evolve more quickly (<24 hours) following inhalational and/or presumed aspiration events.1,9 In acute disease, sepsis syndrome is common; >50% of patients are bacteraemic at presentation, and 20% develop septic shock.5 Pulmonary involvement is the most common acute infection in adults (Figure 2), responsible for >50% of presentations, while pneumonia is seen in approximately 20% of paediatric cases.8 Presentations in children more frequently have skin involvement (60%), compared with 13% in adults.5,10 Cutaneous melioidosis often presents with a solitary lesion at the site of inoculation, typically unresponsive to standard antibiotics.5,6 Visceral abscesses are commonly documented in the spleen, liver, adrenals and kidneys,5 and, chiefly in Australia, prostatic abscesses occur in approximately 18% of males (Figure 3).
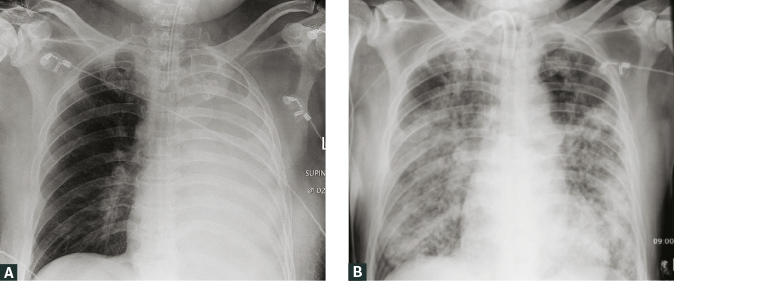
Figure 2. A. Plain chest radiograph from an Aboriginal man, aged 54 years, with poorly controlled type 2 diabetes showing complete left lung consolidation requiring intubation on presentation; B. Follow-up plain chest radiograph in hospital on day 28, showing progressive acute pulmonary melioidosis with extensive bilateral alveolar infiltrates
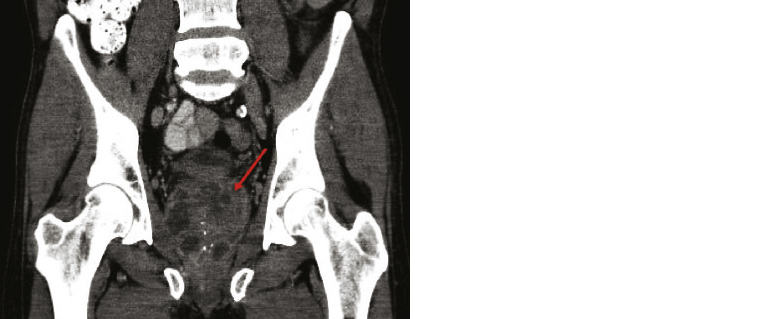
Figure 3. Coronal computed tomography scan of a Caucasian man, aged 62 years, with a large prostatic abscess (arrow), after recent travel to Thailand that resulted in acute disseminated melioidosis
Encephalomyelitis is an infrequent but potentially devastating complication that presents more commonly in Australia (approximately 4% of cases) and frequently involves the brainstem.1 Central nervous system disease is seen outside of Australia but is usually attributable to intracerebral abscesses secondary to bacteraemic spread.11 Osteomyelitis and septic arthritis are described, either due to penetrating injuries or through haematogenous dissemination. Mycotic aneurysms, pericarditis, mediastinal masses and scrotal abscesses are rare clinical manifestations of melioidosis.1,5
Most patients develop acute melioidosis following recent infection, but approximately 11% of cases develop chronic melioidosis (symptomatic for >2 months), usually with constitutional symptoms and often upper lobe infiltrates. Approximately 4% of cases develop pulmonary reactivation of latent disease, sometimes decades following the initial infection.5 Relapse of primary infection (or recrudescence) can occur.2,3,5 The clinical features of melioidosis are summarised in Table 1.
Diagnosis
Melioidosis is underdiagnosed because of its variable non-specific clinical manifestations, limited awareness of the disease and misidentification of B. pseudomallei, particularly in inexperienced laboratories.1,3,5 Acute infections are often misdiagnosed as community-acquired pneumonia, localised skin infection or septicaemic shock. Conversely, chronic lung infection often resembles pulmonary tuberculosis.5
Culture of B. pseudomallei from clinical specimens remains the mainstay of diagnosis, with slow growth on standard blood/MacConkey agar media. Identification of B. pseudomallei using traditional commercial biochemical identification systems is inconsistent,3 resulting in it often being misidentified as Pseudomonas or other Burkholderia species, particularly outside endemic areas.1–3 Isolation is enhanced by inoculating non-sterile specimens onto selective media (eg Ashdown’s agar), which facilitates detection of colonies.1,3 Where available, matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry systems with advanced database libraries can provide rapid, accurate identification of B. pseudomallei.3
Serologic assays have limited clinical use because of high background antibody positivity in endemic regions and false negative results in early disease.3 A lateral flow immunoassay shows promise in Thailand but is currently unavailable in Australia.3
Management
Acute melioidosis typically requires rapid clinical triage, resuscitation and transfer for inpatient management in an acute hospital setting. Nonetheless the primary care physician plays a key role in initial patient assessment, early disease recognition and patient stabilisation, particularly in rural locations. They are responsible for obtaining diagnostic samples (including blood cultures), in combination with early commencement of antimicrobial therapy and prompt transfer. However, some acute localised infections are managed in the community.1,2 Antimicrobial treatment of melioidosis is summarised in Table 2.2,12,13 Once the patient has shown a satisfactory clinical response, outpatient parenteral antimicrobial therapy may be considered during the intensive phase of therapy,13 necessitating appropriate clinical governance by a primary care physician. Following intensive therapy, antibiotic management during the eradication phase is best managed by a general practitioner (GP) to facilitate antimicrobial adherence, limit drug toxicity and detect early treatment failure.12
| Table 2. Management of melioidosis* |
| Intensive therapy |
Initiated on diagnosis to manage high bacterial loads and septicaemia12,13 |
Antimicrobial options
|
Meropenem: IV 25 mg/kg to 1 g tds, IV 50 mg/kg to 2 g tds for CNS disease12,13 |
| Ceftazidime: IV 50 mg/kg to 2 g, 6-hourly. Continuous IV infusion given as a 6 g/day, may facilitate early discharge in suitable patients via OPAT12,13,† |
| TMP-SMX 320/1600 mg bid, folic acid (0.1 mg/kg up to 5 mg in children) daily, may be added for severe disease13,15 |
| Duration |
IV therapy for 10–14 days for isolated pulmonary/cutaneous disease/bacteraemia without focus12,13 |
| IV therapy for ≥4–8 weeks in severe disease/clinical deterioration/complicated pneumonia, deep-seated infection, bone/joint infection, neurological disease12,13 |
| Adjunctive therapy |
Surgical procedures for sanctuary sites less responsive to antimicrobial therapy13 |
| Inconclusive evidence for granulocyte colony stimulating factor2 |
| Eradication therapy |
Instituted immediately following intensive therapy to prevent relapse |
| Antimicrobial options |
Oral TMP-SMX 320/1600 mg bid, monotherapy12,13 |
| Doxycycline in case of TMP-SMX intolerance or adverse drug reaction12 |
| Amoxicillin/clavulanic acid reserved as third line given an association with treatment failure and relapse13 |
| Duration |
Duration usually 3–6 months, guided by clinical response13 |
| Monitoring |
Patient adherence, haematology, biochemistry and markers of disease recrudescence13 |
| Relapse |
Inadequate treatment duration, non-adherence, severe disease, persistent infection |
| Management |
Identify and address the cause of relapse13 |
| Repeat antimicrobial susceptibility testing13 |
| Re-initiation of bactericidal intensive phase treatment13 |
| Post-exposure prophylaxis |
Serious laboratory misadventure, assess need for prophylaxis (limited data)13 |
| Liaise with infectious diseases physician and/or clinical microbiologist |
| Antimicrobial options |
Oral TMP-SMX 320/1600 mg bid13 |
| Amoxicillin/clavulanic acid 875/125 mg bid13 |
| Duration |
Complete 21 days therapy, monitor for signs of infection13 |
*Management of melioidosis is often complex and expert input from an infectious diseases physician and/or clinical microbiologist is recommended for most cases
†OPAT programs facilitate delivery of intravenous antimicrobial therapy in the outpatient setting, as an alternative to inpatient care12
bid, twice daily; CNS, central nervous system; IV, intravenous; OPAT, outpatient parenteral antimicrobial therapy; tds, three times daily; TMP-SMX, trimethoprim-sulfamethoxazole |
Prognosis
The mortality from acute melioidosis is 20–50% worldwide.3 Mortality is even higher (>50%) in resource-poor settings with limited access to modern diagnostic and intensive care unit (ICU) facilities, particularly in people who have significant comorbidities.3 Outcome correlates with rapid disease recognition, appropriate antibiotics and chronic ill-health. Host comorbidities are key factors in determining disease severity,1–3 although these are almost exclusively found in adults, rather than children.5–7 Thai data demonstrated that independent risk factors for death and treatment failure are bacteraemia (odds ratio [OR]: 2.9), respiratory failure (OR: 6.7), renal failure (OR: 3.1) and age >50 years (OR: 2.0); these results were corroborated by observational Australian data.5,14 Although the data is sparse, the prognosis for neurologic melioidosis is generally guarded.11 Outcomes for patients with chronic melioidosis are much better than for those with acute disease, while there is limited prognostic data for reactivation of latent infection.1–3
Public health and biosafety concerns
There has been a recent upsurge in research into B. pseudomallei, arguably driven by its tier 1 select agent (ie potential bio-warfare pathogen) status.3,13 No human vaccines are available.3,13 Disease notification provides opportunities to mitigate public health risks. For example, annual public health alerts with basic education in endemic areas may prevent infections during high-risk periods, particularly for patients with diabetes or other immunodeficiency disorders.3 Tourists, particularly those travelling to endemic areas who are at high risk, should be counselled to minimise contact with soil and surface water, with protective footwear and dressings protecting abrasions and cuts. Highly susceptible individuals (eg people with cystic fibrosis) should be counselled to defer travel during hyperendemic periods, such as during monsoonal rains.
Case report
A healthy stockman, aged 30 years, was referred from the Pilbara region in northern WA with a three-week history of malaise, low-grade fevers, fatigue, productive cough and 5 kg weight loss. In the week prior, he reported a persistent diffuse headache together with progressive diplopia. There was no history of recent travel or hazardous alcohol consumption, and no recent heavy rainfalls. The GP noted the combination of systemic symptoms and progressive neurological deterioration. Following a plain chest radiograph demonstrating left apical cavitatory lesion (Figure 4A), the patient was commenced on empiric oral amoxicillin and doxycycline, and urgent transfer to Perth was arranged.
Initial assessment in Perth showed a drowsy febrile man with a stable haemodynamic profile, but rapidly progressive cranial neuropathy inclusive of bilateral abducens nerve palsies, left facial nerve palsy, and deteriorating speech and cough due to a bulbar/lower cranial polyneuropathy. Respiratory, cardiovascular and gastrointestinal examinations were initially normal. Pertinent laboratory investigations on admission showed C-reactive protein 15 mg/L (reference range [RR] <5 mg/L), leukocytosis 11.58 × 109/L (RR 4–11 × 109/L) and hyponatremia 127 mmol/L (RR 134–146 mmol/L). Shortly following admission, ICU transfer was required for monitoring of labile blood pressure and non-invasive ventilation (forced vital capacity [FVC] 75% predicted, with compensated respiratory acidosis), likely due to probable brainstem autonomic dysfunction. Given the patient’s rapidly progressive neurological deterioration, empiric broad-spectrum antibiotic (including meropenem) therapy was commenced.
Contrast-enhanced computed tomography (CT) of the chest confirmed a left apical cavity, and magnetic resonance imaging of the brain demonstrated extensive T2 signal changes extending from the midbrain to cervical cord (Figure 4B), with leptomeningeal enhancement. A lumbar puncture revealed cerebrospinal fluid (CSF) pleocytosis with 37 × 106/L leukocytes (RR <5 × 106/L), 20% neutrophils and 80% lymphocytes, and raised CSF protein 0.87 g/L (RR 0.15–0.45 g/L). Although both the CSF and blood cultures were negative, sputum yielded B. pseudomallei and the patient started induction therapy with higher doses of intravenous meropenem (2 gm tds) and trimethoprim/sulfamethoxazole (TMP-SMX) 320/1600 mg bid (plus folic acid supplementation). Abdominal CT revealed no intra-abdominal or prostatic abscesses. Following four weeks of intensive phase therapy, he continued oral TMP-SMX 320/1600 mg bid eradication therapy for six months, requiring close monitoring of his clinical status, liver and renal function; supervised by the primary care physician. The GP was also instrumental in spearheading the intensive period of neurorehabilitation following initial management in the acute hospital setting. Assessment at completion of therapy showed full neurological and functional recovery, with resolution of his neuro-imaging changes. He returned to full-time work on the cattle station.
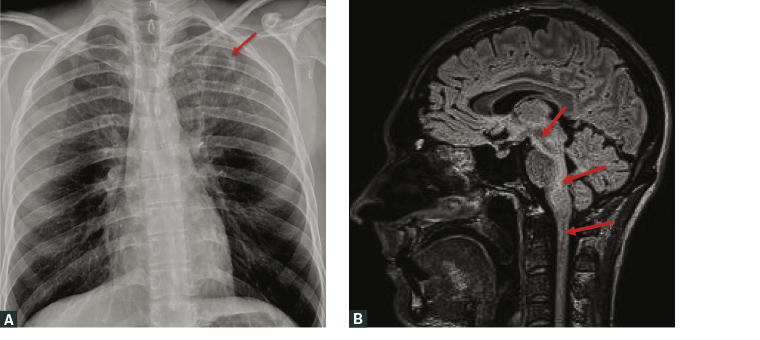
Figure 4. a. Plain chest radiograph showing a 27 mm cystic ovoid cavitating lesion (arrow) within the left lung apex, with surrounding reticular and nodular peribronchovascular opacities; b. Sagittal T2-weighted magnetic resonance imaging showing extensive high-signal change in the midbrain, pons, middle cerebellar peduncles, cerebellum, medulla and superior cervical cord (arrows), plus leptomeningeal enhancement. Not all changes are shown in this image
The regional public health unit investigated the patient’s workplace (spanning approximately 2500 km2), undertaking environmental sampling of local soils and water reservoirs. No source for infection was found, possibly because of the limitations of testing over such an extensive area. The patient’s work colleagues showed no evidence of past serological exposure to B. pseudomallei.
Key points
- Melioidosis is endemic to Northern Australia and South East Asia, particularly after heavy rainfall, although sporadic clusters do occur outside endemic regions.
- Known as the ‘great mimicker’, melioidosis presents with a wide spectrum of disease including isolated cutaneous/pulmonary disease, primary bacteraemia, visceral abscesses or fulminant fatal septicaemia.
- Risk factors for melioidosis include diabetes mellitus, hazardous alcohol use and chronic kidney disease. Less clearly defined independent risk factors include chronic lung disease, malignancy and systemic corticosteroid use.
- GPs play a key role in early diagnosis, which is challenging and requires a high index of suspicion.
- Laboratory culture is central to the diagnosis, with positive cultures considered diagnostic.
- Therapy requires bactericidal induction therapy, with a carbapenem (or ceftazidime) ± TMP-SMX given promptly, followed by TMP-SMX eradication therapy. Choice and duration of therapy is guided by disease severity and organ involvement.
- A human vaccine is not available. Travellers to endemic areas, chiefly high-risk individuals, should be counselled based on an individual risk assessment.