Approximately 2500 patients are diagnosed with type 1 diabetes mellitus (T1DM) each year in Australia.1 The burden of complex management of diabetes and its complications is associated with significant risk of psychological and physical morbidity for those affected by this constant and chronic condition.2 There is strong evidence that intensive glycaemic management in patients with T1DM is associated with a reduced risk of microvascular and macrovascular complications of diabetes, and is also associated with lower all-cause mortality after long-term follow-up.3,4
Prior to the past 20 years, individuals with T1DM were managed with multiple daily injections of insulin, guided by self-monitoring of blood glucose by regular fingerprick testing. Since the introduction of insulin pumps and continuous glucose monitoring (CGM), increasing numbers of individuals are using these systems; however, their use is limited by cost and individual factors. A recent audit reported as few as 12% of individuals with T1DM in Australia were using insulin pump therapy; however, recent advances in technology have resulted in increased uptake of pumps.5 For individuals who continue multiple daily injections, improvements in injectable insulins may be of benefit.
Insulin pumps
Basic principles of insulin pump therapy
Insulin pumps are similar in size to a mobile phone and designed to dose insulin in increments as small as 0.025 units per hour (Figure 1). This allows for precise insulin dosing according to each individual’s requirements. A reservoir is filled with insulin and connected to an infusion set that is inserted into the subcutaneous tissue and changed every 2–3 days. Insulin pumps use rapid-acting insulin analogues to meet the individual’s basal and bolus requirements. When an individual transitions to insulin pump therapy, their injectable insulin doses are used as a guide to determine the initial pump settings.
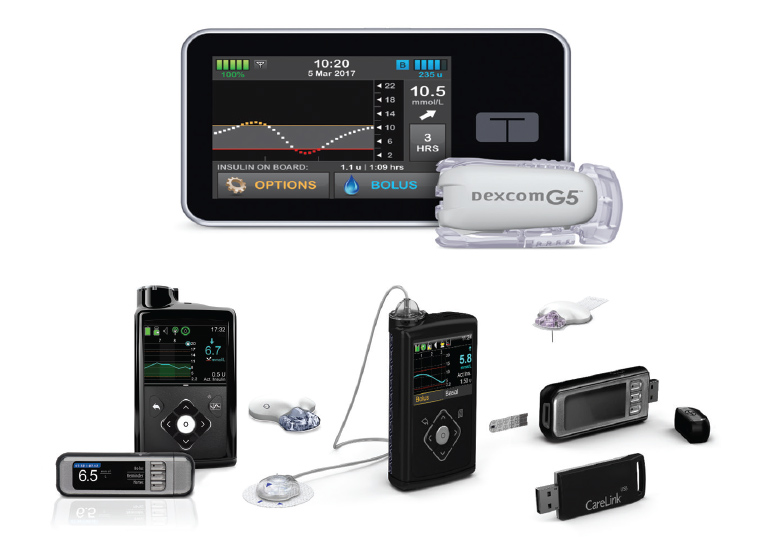
Figure 1. Examples of available insulin pump models that allow integration with continuous glucose monitoring systems30,31
Image of Medtronic pumps reproduced with permission from Medtronic. Copyright ©2018 Medtronic. All rights reserved. Image of T-slim pump reproduced with permission from AMSL Diabetes.
Due to enhanced absorption, insulin doses are reduced by 20–30% when an individual commences pump therapy. The amount by which the dose is reduced depends on the most recent glycated haemoglobin (HbA1c) result and recent glucose readings. As a general rule, approximately 50% of the total daily insulin dose is used as basal insulin and 50% as bolus insulin.6
Basal insulin
Insulin is programmed to be delivered continuously on an hourly rate, and aims to mimic normal insulin production of the pancreas. The infusion rate is often programmed to change at various times during the day and night, with the aim to keep glucose levels stable when the individual is not eating. The basal rate can be temporarily adjusted up or down by the person with T1DM to account for events such as illness or exercise and prevent glucose excursions.
A limitation of once-daily or twice-daily basal insulin injections is that the dose cannot be instantly adjusted to respond to current daily events. In contrast, delivering basal insulin via an insulin pump allows a flexible approach to insulin dosing, based on planned activities and current health status.7
Bolus insulin
Bolus insulin is delivered to correct an elevated glucose level or to manage glucose levels when carbohydrates are eaten. Bolus insulin is calculated by the pump using the following settings:
- insulin to carbohydrate ratio (ICR): the amount of ingested carbohydrate covered by one unit of insulin
- insulin sensitivity factor (ISF): the number of mmol/L by which each unit of insulin will lower glucose levels
- blood glucose target: the pump uses the blood glucose target when calculating correction insulin using the ISF and the active insulin/insulin on board
- active insulin/insulin on board: The amount of insulin still being used by the body from a previous bolus. Insulin pumps compute the active insulin amount before calculating the next correction bolus to reduce the risk of insulin stacking and hypoglycaemia.
Advantages of insulin pump therapy
Continuous subcutaneous insulin infusion pumps have a number of benefits compared with traditional multiple daily injections. One observational study showed an association between insulin pump use and lower all-cause mortality in people with T1DM; however, these study findings may be subjected to confounding as may occur with observational studies.8 Additional studies are required to investigate this issue. A meta-analysis of randomised controlled trials demonstrated a significant reduction in the time spent in hypoglycaemia with pump therapy.9 Blood glucose variability, which may play a role in the development of microvascular complications of diabetes, is significantly reduced in patients who use insulin pump therapy.10 Pump use is also associated with improved lifestyle flexibility, quality of life and satisfaction with diabetes treatment.11 A statistically significant improvement in HbA1c with pumps has consistently been demonstrated in clinical trials, and although this appears modest (approximately 0.3–0.7%), this must be considered in the context of the significant reduction in hypoglycaemia seen with pump therapy.12,13
Disadvantages and safety of insulin pump therapy
Pump therapy is costly, and out of reach financially for most individuals with T1DM. The current funding model for pump therapy in Australia is flawed; as a result, pumps are only practically accessible to individuals with private health insurance and insurers recently announced further restrictions to patients with top hospital cover, which came into effect in early 2019. The full cost of pump devices, covered by private health insurance, ranges from $7362 to $9025.14 Ongoing out-of-pocket patient costs for pump consumables is approximately $30 per month. The out-of-pocket cost for full-time CGM is approximately $3000–$5000 per year.
Insulin pump therapy may be inappropriate or challenging for some people. This highlights the need for a multidisciplinary approach to the initiation of insulin pump therapy. The multidisciplinary team should include a credentialled diabetes educator experienced with insulin pump use, an endocrinologist, a dietitian and a general practitioner. It is essential to ensure each person is assessed for suitability and, if deemed suitable, that they receive high-quality, comprehensive education prior to initiation of pump therapy, and support during pump use.
There are risks associated with insulin pump therapy including pump failure and line issues. These episodes are not infrequent and extensive education regarding troubleshooting is required prior to pump initiation. Additionally, infusion site reactions such as lipohypertrophy can occur in up to 25% of patients, and individuals can experience topical reactions to adhesives used to apply infusion sets. These reactions can often be managed effectively with simple strategies.
Sensor-augmented pump therapy
Sensor-augmented pump therapy refers to automation of insulin delivery via an insulin pump with concurrent use of an insulin pump and a CGM system. There are currently three pump models available in Australia that provide this option:
- Medtronic Veo insulin pump with Medtronic G2 transmitter/Enlite sensor with threshold low-glucose suspend. Insulin delivery is suspended at a prespecified low-glucose threshold (set by the diabetes care team) for reduction in hypoglycaemia.
- Medtronic MiniMed 640G insulin pump with Medtronic G2 transmitter/Enlite sensor with predictive low-glucose management. Insulin delivery is suspended when the blood glucose level is above the prespecified low-glucose threshold and predicted by an algorithm to reach the low-glucose threshold within 30 minutes.
- Medtronic 670G insulin pump with Medtronic G3 transmitter with hybrid closed-loop technology. This system uses complex predictive algorithms to automatically increase, decrease or suspend insulin delivery to keep the glucose level at a specific target glucose of 6.7 mmol/L. Real-world data suggests this results in an increase in time spent in the target glucose range (3.9–10 mmol/L) and a reduction in time spent in hypoglycaemia.15
Switching from optimised multiple daily injections of insulin to sensor-augmented pump therapy can result in significant improvements in HbA1c without an increase in hypoglycaemia.16 The addition of predictive low glucose management systems results in less time in hypoglycaemia and hyperglycaemia.17 Safety of the hybrid closed-loop system has been demonstrated in one study.18
CGM systems
Basic principles of CGM
Traditional CGM works by placing a small electrode (glucose sensor) into the interstitial fluid using an introducer needle. The sensor is connected to a transmitter that can send the data to an insulin pump, a mobile phone or another viewing platform. The sensor is worn for 6–7 days. The individual can view real-time glucose levels, together with trend arrows, which allow timely decision making regarding blood glucose management, whether using insulin pump therapy or multiple daily injections. The glucose value is updated every five minutes, allowing the individual time to respond to glucose trends. As CGM measures interstitial glucose, an ongoing issue is the delay due to the physiological lag between interstitial glucose and blood glucose concentrations, which is particularly problematic at low blood glucose levels.19
CGM enables the generation of an ‘ambulatory glucose profile’ report which assists in guiding management and overcomes limitations in HbA1c as a measure of glycaemic management. There is evidence that CGM leads to improvements in glycaemic management,20,21 a reduction in hypoglycaemia,19 and improved quality of life.21 Use of CGM in people with T1DM during pregnancy has been shown to be associated with improved neonatal outcomes, likely due to a reduction in maternal hyperglycaemia.22
Some continuous glucose sensors can communicate with linked insulin pumps (as above) to adjust insulin delivery according to sensor glucose values. In these models, insulin delivery is suspended if the glucose sensor predicts that hypoglycaemia will occur or if hypoglycaemia has occured, resulting in a reduction in hypoglycaemia.17,23 A ‘hybrid closed-loop’ system is now available, which allows adjustments (up or down) of basal insulin delivery according to sensor glucose via a complex algorithm, without requiring input from the person with diabetes. Bolus insulin delivery is still managed by the individual. Real-world data suggests this results in reduction in hypoglycaemia, and increased time in the target glucose range (3.9–10 mmol/L).15 Characteristics of continuous glucose monitoring devices available in Australia can be found in Table 1.
| Table 1. Characteristics of continuous monitoring devices available in Australia33 |
|
Sensor device
|
Accuracy (%MARD)* |
TGA approved for dosing |
Duration of wear |
Calibration requirement |
Connect to smart phone |
Connect to insulin pump |
Remote monitoring |
Alarm capacity |
Low glucose suspend |
| RETROSPECTIVE |
|
|
|
|
|
|
|
|
|
| Medtronic iPro 2 |
| Enlite sensor and iPro recorder |
11 |
No |
6 days |
2/day |
No |
No |
No |
No |
No |
| REAL-TIME |
|
|
|
|
|
|
|
|
|
| Medtronic |
|
|
|
|
|
|
|
|
|
| Enlite sensor and Guardian 2 link transmitter† |
11 |
No |
6 days |
2/day |
No |
Yes
MiniMed 640G |
No |
Yes |
Yes |
| Enlite sensor and Guardian 3 link transmitter |
9.6 |
No |
7 days |
2/day |
No |
Yes MiniMed 640G or 670G |
No |
Yes |
Yes, plus hybrid closed-loop technology |
| Enlite sensor and MiniLink transmitter‡ |
14 |
No |
6 days |
2/day |
No |
Yes MiniMed Veo |
No |
Yes |
No |
| Enlite sensor and Guardian connect transmitter |
11 |
No |
6 days |
2/day |
Yes |
No |
Yes |
Yes |
No |
| Dexcom |
|
|
|
|
|
|
|
|
|
| Dexcom G4 |
13 |
No |
7 days |
2/day |
No |
Yes
Animas |
Yes |
Yes |
No |
| Dexcom G5 |
9 |
Yes |
7 days |
2/day |
Yes |
Yes
Animas |
Yes |
Yes |
No |
| Abbott |
|
|
|
|
|
|
|
|
|
| FreeStyle Libre |
11.4 |
Yes |
14 days |
None |
No |
No |
No |
No |
No |
*Values should be interpreted with caution as few direct comparative studies within or between device manufacturers are available2–5
†Can only be worn with insulin pump Mini MiniMed 640G, does not connect to other receiver
‡Can only be worn with insulin pump Mini MiniMed Veo, does not connect to other receiver
%MARD, percentage mean absolute relative difference; TGA, Therapeutic Goods Association |
Flash glucose monitoring
Flash glucose monitoring has been taken up with enthusiasm by people with diabetes (Figure 2). This technology allows individuals to check glucose levels without fingerpricking. The FreeStyle Libre is the only device currently available for this purpose in Australia. A sensor attached to a white disc is worn by the patient, usually on the upper arm. Scanning of this sensor by a handheld reader device or mobile phone gives a glucose level, and stores up to eight hours of glucose data which provides an ambulatory glucose profile.24 Real-time flash glucose monitoring was compared with self-monitoring of blood glucose in well-controlled T1DM.25 Mean sensor glucose levels and HbA1c levels did not change but the mean time in hypoglycaemia was significantly reduced in the flash glucose monitoring group.25 Real-time glucose trend data, rather than retrospective analysis of the recordings, were predominantly used for self-adjustments of glycaemic control in this study.

Figure 2. Image of patient using a flash glucose monitoring system32
Reproduced with permission from Abbott Diabetes Care.
CGM systems, other than flash glucose monitoring, are government-subsidised for individuals with T1DM aged under 21 years. The Australian federal government recently announced additional funding for continuous monitoring in women who are planning pregnancy, are pregnant or breastfeeding, and people aged 21 or over with a concession card and high clinical need (eg recurrent, severe hypoglycaemia). Unfortunately, continuous and flash glucose monitoring systems will remain costly (approximately $3000 to $5000 per year) for other adult patient groups. A comparison between self-monitoring, flash glucose monitoring and continuous glucose monitoring can be found in Table 2.
| Table 2. Comparison between self-monitoring of blood glucose, continuous glucose monitoring and flash glucose monitoring technology33 |
| |
Self-monitoring of blood glucose |
Continuous glucose
monitoring |
Flash glucose
monitoring |
| Glucose measurement |
Capillary |
Interstitial |
Interstitial |
| Calibration |
NA |
Twice daily |
Not required |
| Fingerprick required |
Yes |
Yes |
If discrepancy |
| Duration of sensor use |
NA |
6–7 days |
14 days |
| Data updated |
NA |
Every 5 minutes |
When the sensor is scanned with the reader |
| Hypoglycaemic alarm |
No |
Yes |
No |
| Connection to insulin pump |
No |
Yes |
No |
| Connection device |
NA |
Disposable sensor is worn on the abdomen |
Disposable sensor worn on the back of the arm |
| NA, not applicable |
Recently approved insulins
The following insulins have been available on the PBS in Australia for a number of years:
- rapid-acting insulin analogs
- short-acting regular insulin
- intermediate-acting insulin
- mixed insulins
- long-acting insulin.
The range of insulins available in Australia has recently expanded to include the following.
Insulin glargine 300 units/mL
Insulin glargine, a long-acting basal insulin, is contained in commonly used devices at a dose of 100 units/mL. When concentrated to 300 units/mL, the insulin administered is one-third the volume of an equivalent dose of insulin glargine in 100 units/mL preparations, although the pen delivery device is adapted for this. This can result in improved insulin absorption, particularly in patients receiving large insulin doses, but in general glycaemic efficacy is similar.26–28 Devices that deliver insulin glargine 300 units/mL and insulin glargine 100 units/mL are not dose-equivalent, but it is recommended patients are switched dose-for-dose then titrated. This should be considered as a basal insulin in individuals with high basal insulin requirements and/or recurrent hypoglycaemia.
Insulin degludec 70%/insulin aspart 30%
A premixed insulin containing short-acting insulin aspart (30%) and ultra–long acting basal insulin degludec (70%) has become available. It can be administered once or twice daily with the largest meal(s), thus the total number of daily insulin injections may be reduced in some people. The dose is titrated weekly. The rapid-acting component of this premixed insulin means that it has rapid onset of action, with the insulin degludec component providing stable basal coverage with a half-life more than 24 hours. Due to its long half-life, steady state occurs after 2–3 days of administration. Although HbA1c and fasting plasma glucose levels are similar when insulin degludec is compared with insulin detemir, rates of nocturnal hypoglycaemia are significantly lower.29 This insulin should be considered for individuals wishing to reduce the total number of daily injections of insulin, or for those with recurrent hypoglycaemia.
Conclusion
Evolving technology has led to the development of glucose-sensing and insulin-delivery technology that may enhance quality of life, improve glycaemic control and prevent hypoglycaemia in individuals living with T1DM. Unfortunately, uptake of these systems has been limited by financial factors. Individual factors may also play a role in limiting the use of these devices. Multiple daily insulin injections remain a mainstay of therapy in T1DM and the emergence of new, improved insulins may lead to improvements in patients receiving multiple daily injections.