Atrial fibrillation (AF) is the most common recurrent arrhythmia in adult clinical practice and is associated with significant morbidity and mortality.1,2 AF is independently associated with stroke, heart failure and all-cause death.1–3 The prevalence of AF is estimated to be 2–4% in developed countries1,4 and increases with age, but this only reflects clinically detected AF; the true prevalence of AF is suspected to be greater when subclinical or ‘silent’ AF is included.5
Given AF’s varying clinical manifestations, the aim of this article is to distil management concepts into practical recommendations that are useful in general practice.
Risk factors for, and lifestyle management of, atrial fibrillation
Table 1 lists the most clinically relevant risk factors and disease associations for AF. It is important to note that metabolic syndrome and several of its modifiable constituents (abdominal obesity, hypertension, impaired glucose tolerance/type 2 diabetes) are strongly associated with AF.6–8 A number of studies have investigated whether the risk of developing AF can be reduced with exercise; the majority suggest that moderate physical activity is beneficial in reducing AF risk.9 Once AF has been diagnosed, weight loss and aerobic activity have been shown to decrease both the number of AF episodes and symptoms related to AF.8,10 In light of these findings, the National Heart Foundation of Australia’s (NHFA’s) 2018 AF guidelines recommend aerobic exercise and a target body mass index of 27 kg/m2 in patients with AF.9
| Table 1. Risk factors, disease associations and potentially reversible precipitants for atrial fibrillation |
| Risk factors and disease associations |
Potentially reversible precipitants |
- Obesity
- Hypertension
- Type 2 diabetes/impaired glucose tolerance
- Smoking
- Obstructive sleep apnoea
- Coronary artery disease
- Valvular heart disease
- Heart failure
- Chronic kidney disease
|
- Hyperthyroidism
- Alcohol excess
- Electrolyte abnormalities
- Sepsis
|
The patient with newly diagnosed atrial fibrillation
The NHFA’s AF guidelines recommend opportunistic AF screening in patients aged ≥65 years with either radial pulse palpation followed by a 12-lead electrocardiogram (ECG) or a single-lead handheld ECG.9 Therefore, AF can be diagnosed:
- during routine cardiac screening
- because of new onset symptoms
- as an incidental finding in asymptomatic individuals.
Incidental AF is typically diagnosed in two main scenarios: 1) detected on non-invasive cardiac testing (eg echocardiography, 24-hour Holter monitoring, ECG) performed for other clinical reasons, and 2) detected via previously inserted cardiac implantable electronic device (CIED) interrogation (eg implantable loop recorders, pacemakers, defibrillators).
Our suggested approach to patients with newly diagnosed AF is to:
- identify underlying risk factors and reversible precipitants (Table 1)
- characterise structural heart disease that may be associated with AF
- assess the patient for symptoms
- assess and manage ventricular rates while in (and out of) AF
- consider anticoagulation for stroke prevention.
As a first step, it must be ensured that no acute and reversible pathologies are responsible for triggering AF (Table 1, Figure 1); successful implementation of any management strategy in AF is predicated on such factors being identified and addressed. For example, thyrotoxicosis will continue to drive AF with rapid ventricular response despite appropriate medical therapy. Box 1 summarises the essential investigations for patients with newly diagnosed AF; the treating practitioner may choose to do additional testing for reversible precipitants (eg sepsis) depending on the patient’s clinical features. The NHFA recommends polysomnography to diagnose sleep apnoea only for patients with symptomatic AF.9 Transthoracic echocardiography for patients with AF sheds light on:
- the presence of significant mitral stenosis (refer to ‘Anticoagulation for stroke risk’)
- significant occult valvular heart disease (eg mitral or tricuspid regurgitation)
- left ventricular systolic/diastolic function
- left atrial size/volume.
Holter monitoring provides vital information regarding:
- ventricular rate control while in AF and in sinus rhythm (paroxysmal AF)
- ventricular pauses, and whether they are post-reversion from AF to sinus rhythm (Figure 2) or while in AF (may need to consider cardiac pacing if symptomatic).
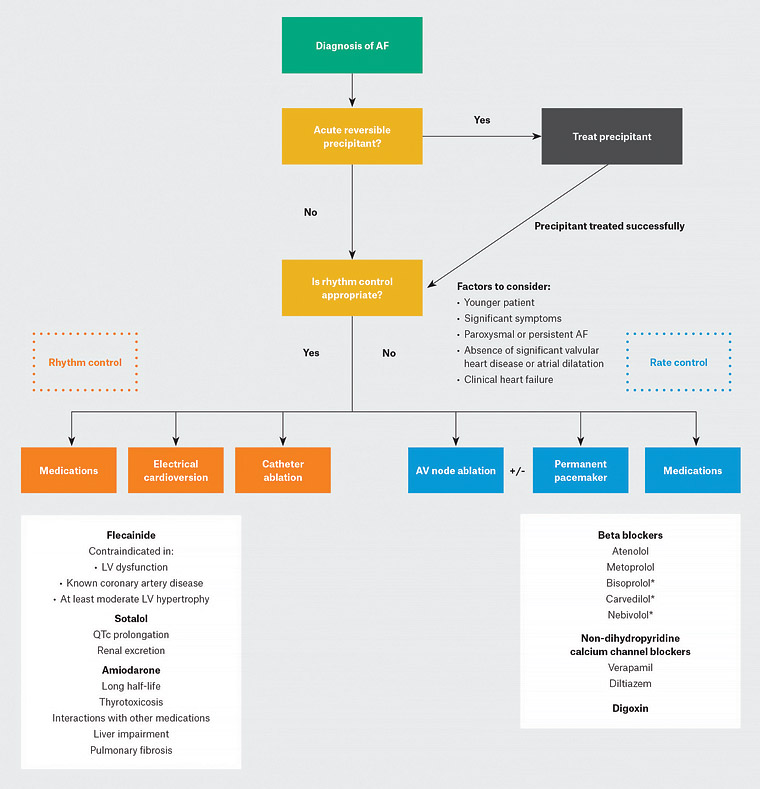
Figure 1. Rate and rhythm control strategies in atrial fibrillation
A summary of factors to consider when deciding whether to pursue a rate or rhythm control strategy, and the pharmacological and procedural therapeutic options available for each strategy (rate control in blue, rhythm control in orange). Common rhythm control medications are summarised on the left, whereas common rate control medications are listed on the right.
*Beta blockers marked with an asterisk are heart failure–specific beta blockers that are often used preferentially for people with AF and clinical heart failure.
AF, atrial fibrillation; AV, atrioventricular; LV, left ventricular
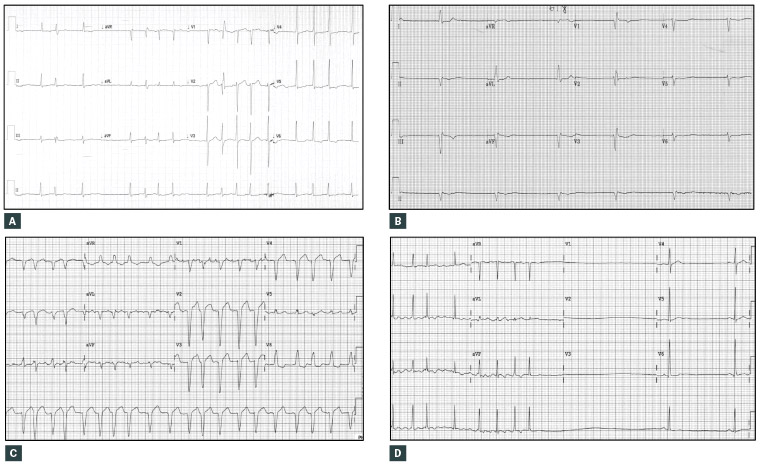
Figure 2. Common patterns of ventricular rates in atrial fibrillation (AF)
A. AF with a controlled ventricular response (mean heart rate 95 beats per minute [bpm]); B. AF with a slow ventricular response (mean heart rate 36 bpm); C. AF with an uncontrolled (or fast) ventricular response (mean heart rate 115 bpm) and left bundle branch block aberrancy; D. AF reverting to sinus bradycardia after a long reversion pause
| Box 1. Routine investigations for newly diagnosed atrial fibrillation |
- Full blood examination
- Urea, electrolytes and creatinine
- Calcium, magnesium and phosphate
- Thyroid-stimulating hormone
- Transthoracic echocardiogram
- 24-hour Holter monitoring
- Polysomnography (in patients with symptomatic atrial fibrillation only)
|
Atrial fibrillation that requires urgent attention
The first consideration for patients with newly diagnosed AF is the presence of any concerning clinical features warranting hospitalisation. Patients with AF and abnormal ventricular rates should be referred to the emergency department in any of the following situations:
- hypotension
- AF with rapid ventricular rates (generally heart rates >110 beats per minute [bpm] or if very symptomatic)
- signs of clinical heart failure
- syncope or presyncope
- rest angina +/– ischaemic ECG changes.
Barring these circumstances, AF can usually be managed safely in the community despite high or low ventricular rates. Data are lacking on the exact classification of ‘acceptable’ ventricular rates;11,12 we recommend that patients with mean heart rates <50 bpm or >110 bpm be evaluated early for arrhythmia-related symptoms or evidence of clinical cardiac failure.
Rate versus rhythm control
Patients with AF can be asymptomatic, symptomatic with normal ventricular rates, and/or symptomatic with only abnormally high or low ventricular rates. When deciding between rhythm control (aiming to maintain sinus rhythm) and rate control (aiming to control ventricular rates while remaining in AF), the aim is to achieve symptom control and ventricular rates that are neither too fast nor too slow. Although both strategies have been shown to be equivalent in terms of long-term outcomes (that is, achieving sinus rhythm does not confer any mortality benefit or reduction in stroke risk when compared with simply controlling ventricular rates in permanent AF where multiple attempts to achieve sinus rhythm have previously failed),13–16 there is one significant proven advantage of rhythm over rate control – relief from AF-related symptoms. Because AF-related symptoms can be quite significant, cardiologists and patients often pursue rhythm control together in the first instance. On average, rhythm control medications halve the risk of AF recurrence9 and are more likely to succeed for patients who:
- are physically active
- have paroxysmal or persistent AF lasting short periods of time
- do not have significant underlying cardiac structural changes (eg severe left atrial dilatation, mitral valve pathology).
The 2016 European Society of Cardiology’s AF management guidelines12 classify AF into:
- paroxysmal (episodes <1 week in duration)
- persistent (episodes >1 week in duration)
- long-term persistent (episodes >12 months in duration)
- permanent (joint decision by physician and patient to accept the presence of AF and focus on rate rather than rhythm control).
This classification reflects the natural history of AF progression for many patients (short paroxysms initially, progressing to more persistent episodes, and eventually into constant ‘permanent’ AF), and is important in understanding when a rhythm control strategy is more appropriate and likely to succeed. A key paradigm in AF management is that ‘AF begets AF’; a vicious cycle of paroxysms of AF causing structural changes in atrial myocardium and increasing the likelihood of further AF episodes has been established as an important mechanism explaining the natural history of AF.17,18 Rhythm control aims to ‘interrupt’ this cycle by establishing and maintaining sinus rhythm early, and is more likely to be effective the earlier it is instituted in the natural history of AF, especially for patients who are engaging in positive lifestyle behaviours.
Rhythm control is also pursued for patients with cardiomyopathy secondary to AF with rapid ventricular response and for susceptible individuals for whom AF may precipitate acute haemodynamic deterioration because of cardiac comorbidities (eg significant aortic stenosis, left ventricular systolic dysfunction, hypertensive heart disease).
Rate and rhythm control strategies
Figure 1 summarises rate and rhythm control strategies. Catheter ablation for AF is established as a rhythm control option but only for symptomatic patients with paroxysmal/persistent AF who are refractory/intolerant to at least one rhythm control medication. It is more effective at reducing symptoms and maintaining sinus rhythm in these patients than continuing medications;9 patients may require more than one procedure to achieve symptom control, with success rates approaching 90% after two procedures.12 Catheter ablation carries a small but not insignificant risk of complications.19
Atrial fibrillation and heart failure
Heart failure and AF coincide for many patients; it can be difficult to tease out which is the cause and which is the effect. Patients with cardiomyopathy secondary to other causes (eg ischaemic or non-ischaemic cardiomyopathy) go on to develop AF, while others develop a cardiomyopathy secondary to AF with rapid ventricular response.11,12 Because AF is often poorly tolerated by heart failure patients in terms of symptom burden and risk of decompensation, one must consider rhythm control early in the treatment of a patient with signs of clinical heart failure and AF.
Anticoagulation for stroke risk
Stroke prevention is a major aspect of AF management. The CHA2DS2-VASc score is widely used in clinical practice in Australia to predict stroke risk in AF. The NHFA’s AF guidelines have simplified estimating stroke risk by removing female sex from the risk calculator, leaving CHA2DS2-VA (Congestive heart failure, Hypertension, Age >75 years [2 points], Diabetes, Stroke/transient ischaemic attack [2 points/1 point respectively], Vascular disease, Age >65 years).9 This has resulted in the following recommendations for both sexes:
- CHA2DS2-VA = 0: Oral anticoagulants (OACs) are not recommended
- CHA2DS2-VA = 1: OACs should be considered
- CHA2DS2-VA = 2: OACs are recommended.
The newer OACs and warfarin have been discussed extensively in recent Australian review articles;20,21 the above recommendations are applicable for patients with both AF and atrial flutter, and hold true irrespective of rate/rhythm control. Further, in the special case of ‘valvular AF’ (defined as the presence of a mechanical heart valve or moderate-to-severe mitral stenosis),12 warfarin therapy is the only effective recommended treatment option, and the newer OACs should not be used. When AF is detected incidentally on routine CIED interrogation, anticoagulation is probably warranted for episodes of AF longer than 24 hours, but this is an ongoing area of clinical research with conflicting evidence.9
Atrial fibrillation and percutaneous coronary intervention: ‘Triple therapy’
Not infrequently, patients have indications for both anticoagulation (to prevent thromboembolism) and antiplatelet agents (to prevent stent thrombosis); in these situations, the medication regimen used needs careful tailoring to the individual patient. Factors that need to be considered include the absolute risk of stroke, the nature of the patient’s coronary disease (eg coronary stenting for stable angina versus an ST-elevation myocardial infarct) and the patient’s bleeding risk. The use of risk stratification calculators such as HAS-BLED are useful: Hypertension, Abnormal renal or liver function (1 point each), Stroke, Bleeding history, Labile international normalised ratios, Elderly (age >65 years), Drugs (antiplatelet agents/nonsteroidal anti-inflammatory drugs) or alcohol (1 point each).22 Some patients are prescribed an anticoagulant and two antiplatelet agents (‘triple therapy’) immediately after percutaneous coronary intervention (PCI), while others are suitable for treatment with an anticoagulant plus a single antiplatelet agent.9 However, several authoritative guidelines agree that patients can usually be safely managed with OACs only (ie no antiplatelet therapy) 12 months after PCI.9,11,12
Key points
- Identify and treat underlying clinical drivers of AF.
- Achieve an appropriate heart rate (>50 bpm and <110 bpm) with either rate or rhythm control to minimise symptoms of AF.
- Diagnose and treat concomitant cardiac pathology such as heart failure and valvular heart disease.
- Consider anticoagulation to reduce stroke risk.
- Refer for specialist cardiology input when concomitant cardiac pathology and/or slow/rapid heart rates are present or persistent.