Gout is a common and important disease that results from an elevation in serum urate. When super-saturation is reached, monosodium urate (MSU) crystals are formed and can deposit in joints and surrounding tissues. MSU crystals are the inflammatory trigger for gout flares, which cause the affected joint to become red, hot, swollen and extremely painful. Inadequately treated gout leads to recurrent gout flares, formation of tophi (accumulation of MSU crystals in joints and soft tissues) and joint damage. Time off work, poor health-related quality of life and disability are common with recurrent gout flares.1 Gout flares are a major concern for people with gout.2
The majority of people with gout are managed in primary care;3 therefore, strategies to improve gout management in primary care are important. Results from studies of primary care suggest that management of gout may be suboptimal. Rates of urate-lowering therapy (ULT) prescription have been reported to be between 25% and 73% and achieving target serum urate between 41% and 70%.4–6 Lifestyle modification has been identified as an important aspect of gout management by general practitioners (GPs) and failure to adhere to recommended lifestyle changes is a key barrier to effective management.7 Other key barriers to optimal gout care include the perception of gout as an acute condition that only requires treatment for the acute episode, rather than a chronic condition in which the acute episodes can be prevented with effective long-term ULT; the medical complexity of many people with gout because of the presence of comorbidities; and poor knowledge about optimal gout management.8
In addition to being the setting where most people with gout are managed, general practice is well placed to provide the screening and management of comorbidities associated with gout as recommended by international guidelines.9–11 However, studies report low rates of screening, for example, a UK study in primary care recorded screening rates for hypertension, hyperlipidaemia, diabetes and renal function of 26%, 5%, 6% and 21%, respectively.12
There has been a recent focus on improving the management of gout in primary care. This has involved both nurse-led and pharmacy-based care. Data from a community-based study in the UK that compared GP and nurse-led care revealed significantly more people achieved target serum urate (29% versus 95%, respectively) and fewer gout flares (mean [standard deviation] flares 0.94 [2.03] versus 0.33 [0.93]) at two years in the nurse-led arm.13 Pharmacy-based models of care trialled in the USA for people with gout have not been as effective, with high numbers of drop-outs.14 While GP-based interventions have had impressive results in the clinical trial setting it remains to be determined whether such improvements can be replicated in routine clinical care.
The aims of this audit were to determine whether the introduction of a structured ‘gout package of care’ based on current American College of Rheumatology and European League Against Rheumatism gout management guidelines9–11 resulted in more people with gout receiving ULT, more people achieving target serum urate, and improved rates of screening for comorbidities associated with gout in a rural primary care setting. Initial results have been published in abstract form15 and in this article we outline the full more detailed description of the methods and analysis.
Methods
Ethical approval was provided by the New Zealand Health and Disability Ethics Committee (14/CEN/163). The audit was undertaken at the Kaikōura Medical Centre, New Zealand, which is 190 km north of Christchurch. This medical centre services a population of approximately 3600 and is the only medical practice within the area. The nearest secondary care facility is in Christchurch. The practice has two permanent GPs in addition to at least two locum GPs, and a number of practice nurses as well as community links through the Whanau Ora nurse from the local Māori Health provider. Of those registered at the practice, 571 (15.9%) identify as Māori, the indigenous people of New Zealand and a group with a high prevalence of gout.
An audit was undertaken of all people with gout enrolled at the Kaikōura Medical Centre between 1 January 2012 and 31 December 2012, two years prior to the introduction of the gout package of care, to avoid the Hawthorne effect. People with gout were identified from the practice electronic medical record either by having a coded diagnosis of gout, having received at least one prescription for ULT for gout or having received colchicine for management of a gout flare. Medical records were reviewed for the following information: demographics, medications including treatments for gout, evidence for screening for comorbidities within five years including glycated haemoglobin, blood pressure, lipids, kidney function, weight, number of times serum urate was checked in the 12-month period, number of allopurinol prescriptions and allopurinol dose and evidence of alteration of ULT if serum urate was >0.36 mmol/L (6 mg/dL).
Gout package of care
The package of care was developed to reflect current guidelines in the management of gout9,10 incorporating the following four elements:
- Education: the following topics were identified as important for patient education in a specific nurse-delivered individual education session – lifestyle (including alcohol use, weight management, dietary triggers), importance of ULT and medication adherence, management of gout flares, use of standard written information sheets about gout and the medications used to manage gout.
- A structured approach to treating gout flares with non-steroidal anti-inflammatory drugs, colchicine or corticosteroid depending on the patient’s comorbidities, concomitant medications and preferences.
- A structured approach to ULT, with allopurinol as the first-line medication, and serum urate monitoring to achieve target serum urate (Figure 1), which incorporated referral criteria for a rheumatologist opinion and recommended starting doses of allopurinol.
- Screening of comorbidities associated with gout, including diabetes, hypertension, hyperlipidaemia, chronic kidney disease and obesity.
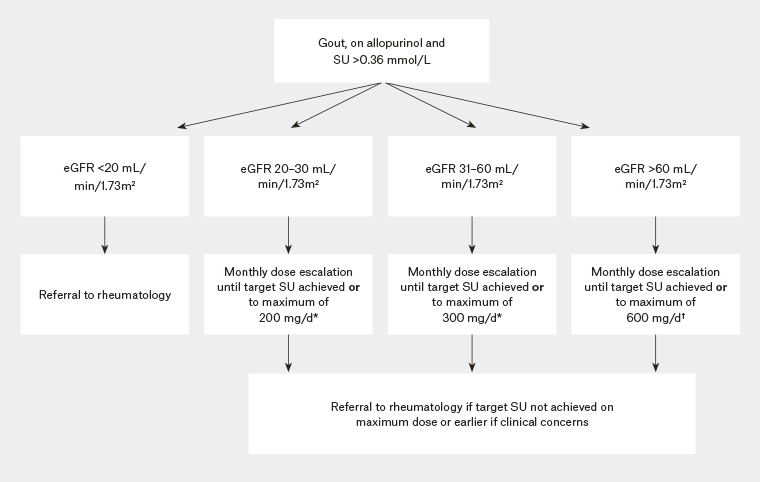
Figure 1. Approach to urate-lowering therapy
*If eGFR <60 mL/min, increase dose by 50 mg/month
†If eGFR >60 mL/min, increase dose by 100 mg/month
eGFR, estimated glomerular filtration rate; SU, serum urate
There was ready access by telephone to a specialist rheumatologist for consultation if required. Suggested referral points were made on the basis of allopurinol dose and renal function (Figure 1) after discussion with the GPs who expressed hesitation over allopurinol dose escalation, particularly in the setting of significant kidney impairment.
A one-day education session was provided by a rheumatologist (LKS) and two clinical nurse specialists, all with expertise in gout management, for all medical staff (nurses and medical practitioners) at the Kaikōura Medical Centre. Standard written information leaflets about gout in both English and Te Reo Māori (Māori language) and a ‘gout card’ to allow people with gout to track their serum urate were provided to the practice. The practice developed a specific electronic record for people with gout to prompt healthcare providers, and wall charts guiding ULT were placed in all clinic rooms (Figure 1). A recall system was established for serum urate monitoring similar to that used for other screening systems in the practice such as international normalised ratio (INR) test recalls for people receiving warfarin. The patients were seen by the medical personnel either with a gout flare or at a routine visit for medication repeats or another problem. Management of the gout flare, some basic information about gout and the gout template was begun by the GP. They referred the patient with gout for blood tests and the more in-depth education package provided by the practice nurses. The follow-up of the blood tests and dose escalation of allopurinol in consultation with the GPs were carried out by the practice nurses and the Whanau Ora (Māori Health) nurse.
The package of care was introduced as best practice for people with gout at the Kaikōura Medical Centre in November 2014. A second audit of the practice was undertaken from 1 February 2015 to 31 January 2016 to determine whether the best practice model had improved the management of gout.
Statistics
Demographic and clinical variables were statistically compared between 2012 and 2015 using Student t tests or Mann–Whitney U tests and chi-square or Fisher’s exact tests as appropriate. A P value of <0.05 was taken to indicate statistical significance.
Results
Demographics and gout education
In 2012, 120 people were identified with gout; in 2015, 171 people were identified with gout. The demographics are detailed in Table 1. There were fewer individuals who identified as Māori than expected.
| Table 1. Basic demographics, comorbidities and concomitant medications |
| |
2012 (n = 120) |
2015 (n = 171) |
P value |
| Percentage of practice population |
3.3% |
4.7% |
|
| Male* |
101 (84.2%) |
142 (83%) |
0.80 |
| Age (years)† |
64.6 (14.5) |
65.7 (13.8) |
0.49 |
Ethnicity*
European
Māori/Pacific Island
Other |
102 (85%)
18 (15%)
0 |
142 (83.0%)
28 (16.4%)
1 (0.6%) |
0.76 |
| Duration of gout (years)‡ |
6.9 (0–34) |
7.4 (0–37) |
0.61 |
| Screening for comorbidities |
| Body mass index (kg/m2)* |
53 (44.2%) |
117 (68.4%) |
<0.001 |
| Mean (range) |
31.1 (18.4–55.2) |
31.4 (14.9–54.6) |
0.52 |
| HbA1c* |
62 (51.7%) |
149 (87.1%) |
<0.001 |
| Mean (range) |
42.9 (31–67) |
39.8 (23–96) |
0.02 |
| Blood pressure (mmHg)* |
105 (87.5%) |
163 (95.3%) |
0.02 |
| Systolic mean (range) |
134 (88–184) |
137 (100–194) |
0.12 |
| Diastolic mean (range) |
80 (50–102) |
78 (30–107) |
0.29 |
| Lipids* |
97 (80.8%) |
145 (84.8%) |
0.37 |
| Total cholesterol mean (range) |
5.2 (2.8–8.4) |
5.1 (3.1–7.6) |
0.45 |
| HDL mean (range) |
1.2 (0.34–2.19) |
1.2 (0.6–2.3) |
0.17 |
| LDL mean (range) |
3.16 (0.7–6.3) |
2.97 (1.4–4.9) |
0.14 |
| Ratio mean (range) |
4.67 (1.0–10.5) |
4.36 (2.2–8.1) |
0.06 |
| Creatinine (μmol/L)* |
110 (91.7%) |
157 (91.8%) |
0.96 |
| Mean (standard deviation) |
98.9 (67–274) |
107.4 (61–413) |
0.10 |
*Number (percentage)
†Mean (standard deviation)
‡Mean (range)
HbA1c, glycated haemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein |
Urate-lowering therapy
Details of ULT are outlined in Table 2. The median (interquartile range) number of prescriptions per individual increased between 2012 and 2015 (1 [0–4] versus 3 [0–4]; P <0.001; Figure 2A). There was an increase in the number of individuals commenced on ≤100 mg daily and a corresponding decrease in the number commenced on ≥200 mg daily (P <0.001; Figure 2B). The use of colchicine and subsequent use of allopurinol is outlined in Table 2.
| Table 2. Urate-lowering therapy |
| |
2012 (n = 120) |
2015 (n = 171) |
P value |
| Prescribed ULT* |
79 (65.8%) |
127 (74.3%) |
0.12 |
| Number of prescriptions for allopurinol† |
1 (0–4) |
3 (0–4) |
<0.001 |
| Started ULT* |
22 (18.3%) |
43 (25.1%) |
0.17 |
| Number of patients with at least one test for serum urate* |
67 (55.8%) |
133 (77.8%) |
<0.001 |
| Number times serum urate tested in audit year† |
1 (0–3) |
2 (0–10) |
<0.001 |
*Number (percentage)
†Median (range)
ULT, urate-lowering therapy |
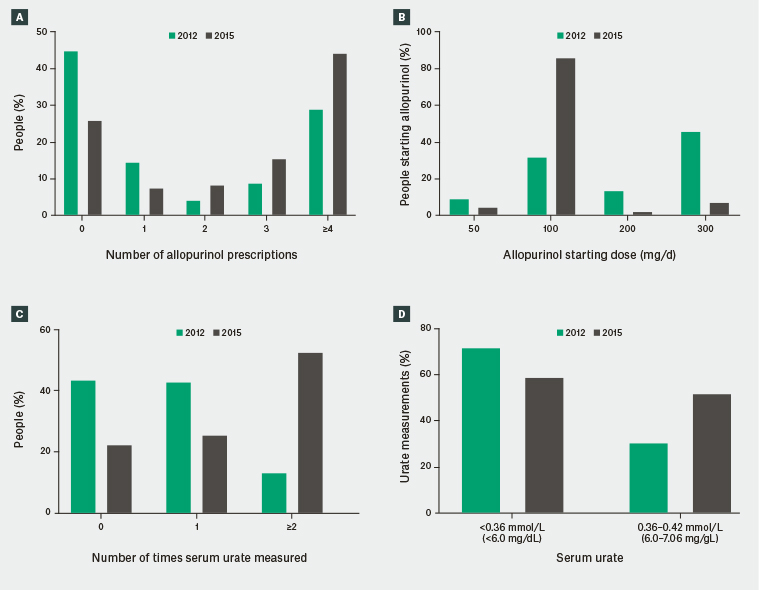
Figure 2. Differences between 2012 and 2015
A. Number of allopurinol prescriptions; B. Allopurinol starting dose; C. Serum urate measurement; D. Serum urate levels
Serum urate
In 2012, 67 out of 120 individuals (55.8%) and in 2015, 133 out of 171 individuals (77.8%) had at least one serum urate measurement taken (P <0.001). There was a statistically significant increase in the number of times each patient had serum urate tested between 2012 and 2015 (median [range] 1 [0–3] versus 2 [0–10] respectively; P <0.001) (Figure 2C). Of those individuals who had at least one serum urate measurement, the number of individuals who were never at target urate was 43 out of 67 (64.2%) in 2012 in comparison to 52 out of 133 (39.1%) in 2015 (P = 0.001).
In 2012, 63 out of 88 (71.6%) serum urate measurement tests were ≥0.36 mmol/L (6 mg/dL) and, of those,19 out of 63 (30.2%) were between 0.36 mmol/L and 0.42 mmol/L (upper limit of normal for urate; 6.0–7.06 mg/dL). In 2015, 265 out of 450 (58.9%) serum urate measurements were ≥0.36 mmol/L (6 mg/dL; P = 0.025 when compared with 2012) and, of those, 137 out of 265 (51.7%) were between 0.36 mmol/L and 0.42 mmol/L (6.0–7.06 mg/dL; P = 0.002 when compared with 2012; Figure 2D).
Allopurinol dose escalation
Of the 67 individuals who had a serum urate measured in 2012, none was dose escalated. Twenty-one out of 67 individuals (31.3%) who did not have an increase had serum urate <0.36 mmol/L (6 mg/dL). Of the 133 individuals who had a serum urate measured in 2015, 34 (25.6%) had at least one increase in allopurinol dose. Twenty-eight out of 99 individuals (28.3%) who did not have an increase in allopurinol dose were at target urate for all measurements and a further 12 (12.1%) had serum urate ≤0.42 mmol/L (7.06 mg/dL; upper limit normal range) for all observations.
Comorbidity screening
Screening for important comorbidities associated with gout improved between 2012 and 2015 (Table 1).
Discussion
This real-life model of gout management in primary care has shown that improvements in adherence to gout management guidelines can be made with a relatively simple package of care such as that provided in this program. An increase in the number of people with gout is likely to reflect increased awareness of the need to manage and document gout within the practice and community awareness that gout could be managed with more than just over-the-counter medications through word of mouth in a small community.
There were a number of positive outcomes observed in this audit, including an increase in prescriptions for ULT, increased screening for comorbidities, increased documentation that education about gout had occurred and a reduction in the number of people never at target serum urate. This was achieved with the provision of a modest package of care implemented in primary care.
Allopurinol dose escalation using the ‘treat-to-target’ serum urate strategy proved challenging. A number of factors are likely to have influenced this. First, the serum urate lower limit of ‘normal’ (0.42 mmol/L [7.06 mg/dL]) is higher than the currently recommended serum urate treatment target for people with gout and hence laboratory results falling within the range 0.36–0.42 mmol/L (6.0–7.06 mg/dL) are not highlighted as ‘abnormal’ and may be signed off without action if there is no clear indication that the patient has gout or that the target is lower in people with gout. Local laboratories should be encouraged to highlight the target serum urate for people with gout when reporting results. Second, the ‘optimal’ target remains debated, particularly in light of the American College of Physicians’ guidelines that advocate a ‘treat-to-symptom’ rather than a ‘treat-to-target urate’ approach in the long-term management of gout,16 acknowledging that these were published after the audit period. On the basis of long-term observational data and some shorter-term randomised controlled trials using an ecological study design, serum urate <0.36 mmol/L (6 mg/dL) has been shown to be associated with fewer gout flares.17 However, it is possible that in people with a serum urate above target (ie >0.36 mmol/L [6 mg/dL]) but below the point of saturation at physiological temperature and pH (ie <0.42 mmol/L [7.06 mg/dL]), further increasing ULT to lower the serum urate does not provide improvement in patient-important outcomes such as gout flares. However, in people with tophi, the reduction in size and/or number of tophi may take longer given the association between urate and speed of tophus reduction.18 Further clinical trials of sufficient duration and size using gout flares as the primary outcome measure, rather than serum urate, will be required to determine whether the currently recommended serum urate targets are appropriate for all people with gout. In this regard, the proposed definition of ‘remission’ in gout demands that all the following must be fulfilled: serum urate <0.36 mmol/L (6 mg/dL) at least twice in the preceding 12 months; no tophi; no gout flares in the preceding 12 months; pain due to gout <2 out of 10 in the preceding 12 months and no values >2 out of 10, and patient global assessment of less than two out of 10 in the preceding 12 months.19 However, there may be resistance by both healthcare professionals and people with gout to increase treatment if all criteria are fulfilled except the serum urate criterion. Such patient and healthcare practitioner factors are not possible to be teased out from this audit. For the group of individuals for whom there have been no gout flares for many years and there is no evidence of tophi or radiological damage, it may be appropriate to review the diagnosis and consider whether long-term ULT continues to be appropriate where there may have been diagnostic uncertainty. However, trials of de-escalation of therapy are sparse but suggest that there will be a symptom-free period before gout recurs after withdrawal of therapy.20
Gout management in primary care can be challenging, particularly in the frequently encountered context of patients whose gout is one of several comorbidities. Guideline recommendations for such patients’ gout management must be considered alongside those of other single-condition guidelines. This is potentially onerous both for patient and provider and there is uncertainty about whether applying the recommendations of multiple guidelines simultaneously leads to net benefit for an individual.21,22 Furthermore, patients who already take multiple long-term medications may be reluctant to commence or increase their medication burden with ULT, even when this is indicated and offered, and their providers may be concerned about causing or exacerbating polypharmacy in such patients. In this audit it was not possible to determine whether the option of starting or increasing ULT had been discussed and was either declined by the patient or not considered clinically appropriate for some reason. The increase in the number of people who had their dose of allopurinol increased between the two audits most likely reflects a benefit of the package of care with a recognition that gout should be treated to target serum urate. The importance of education about gout can also not be underestimated as it is important that people with gout understand the rationale for treatment and the difference between the short-term management of gout flares and long-term need for ULT. Such understanding is likely to have a clinically important effect on adherence. The presence of comorbidities, while bringing patients into more frequent contact with their healthcare providers, may mean that gout management is afforded a lower priority than their other conditions, and the need for frequent consultations may impose financial and time barriers to the provision of optimal gout care. This is particularly important when allopurinol is prescribed as the allopurinol dose-escalation strategy is time consuming and intensive for both the patient and the healthcare providers. Easier and more practical ways to dose escalate allopurinol are required such as dedicated nurses or nurse practitioners and standing orders for allopurinol dose escalation. There is unlikely to be one process that works for every practice and individual primary care practitioners will need to identify the most effective way of ensuring dose escalation occurs if required within their practice framework.
Finally, many people with gout only present with acute symptoms and not when the disease is well controlled. Of some concern is the low number of Māori included, suggesting that access to or presentation to a healthcare professional for gout care remains a significant issue.
There are several limitations to this audit. First, it was undertaken in a single centre and was an uncontrolled before and after audit of ‘best practice’. However, this is also one of the strengths of the audit in that it reflects real-life clinical care and shows the challenges of managing a chronic condition within a busy primary care setting. Second, patient-important outcomes such as the number of gout flares and reduction in tophi were not collected. However, it is important to recognise that the use of patient-important features as outcome measures in studies is challenging as they require very large sample sizes and long-term follow-up (>12 months) for a benefit to be observed.17 Thus, surrogate markers such as serum urate have generally been accepted as primary outcomes in gout studies, including in clinical trials for the registration of new ULTs by the Food and Drug Administration. Audit is likely not strong enough to account for the effect of confounders and other factors that may have influenced the comparative findings between 2012 and 2015. Finally, whether statistical significance is associated with clinical significance remains unclear.
Conclusion
A structured package of care may improve adherence to gout management guidelines in primary care. However, the package of care needs to be simple, easy to follow, adapted for local conditions, incorporate an increased role for practice nurses, and have ready access to specialist advice if required.