With the continued rising incidence of skin cancers in Australia, the need for early detection and timely management is paramount.1,2 General practitioners (GPs) are at the front line of skin cancer detection, given they have more than one million patient consultations per year for skin cancer. Patients present to their GPs for regular, unscheduled and opportunistic skin checks, which, in the reality of primary care, may be difficult to achieve within time pressures.
The ability to confidently diagnose benign and malignant skin lesions is influenced by experience and knowledge, given the spectrum of clinical presentations that both malignant and benign lesions may exhibit. Prior to conducting a full skin examination, it is useful to know what one would expect to find in that particular patient. This is guided by age, site of involvement, Fitzpatrick skin type and consideration of risk factors for skin cancer development. Uncertainty can lead to unnecessary intervention and overtreatment.
Lesions of suspicion, particularly with corroborating histories, warrant biopsies. Histological evaluation will yield three possible diagnoses for a skin lesion: clearly benign, clearly malignant or too close to call. The course of action that ensues is determined by this pivotal decision.
In this article we discuss key elements of a skin examination and some recent advancements for more sophisticated surveillance techniques. We also provide an exploration of some commonly encountered malignant skin lesions in general practice to assist with confidence in the diagnostic process and basic principles of management.
Approach for conducting a skin check
Skin checks are critical for comprehensive assessment and examination for evidence of skin cancer. While routine skin cancer screening is not advocated,3 it largely falls within the realm of primary care to stratify and understand which patients require routine checks in accordance with their presenting risk factors. Table 1 provides a framework on stratifying certain characteristics of patients.
| Table 1. Stratifying risk of skin cancer |
| High risk |
Genetic/non-modifiable |
- Fitzpatrick skin type I–II22
- Red hair
- Family history of melanoma
- Personal history of skin cancer (either melanoma or non-melanoma skin cancer [NMSC])
- Genetic syndromes
|
Encourage 3–4-monthly
self-checks with 6–12‑monthly skin checks with a doctor9,23 |
| Environmental |
- Immunosuppression (renal transplant)
|
| Examination findings |
- >100 naevi (>10 atypical naevi)
- >20 solar keratoses
|
| Increased risk |
Genetic/non-modifiable |
- Fitzpatrick skin type I–II22
- Fair complexion with tendency to burn
- Family history of NMSC
- Male
- Increasing age
|
Encourage 3–6-monthly self-checks with opportunistic checks with a doctor24 |
| Environmental |
- Outdoor work with high levels of ultraviolet exposure
- Solarium use
- Multiple episodes of sunburn with blistering
|
| Examination findings |
- <20 solar keratoses
- Presence of freckles
|
| Average-to-low risk |
Genetic/non-modifiable |
- Fitzpatrick skin type III–VI22
- Olive-darker complexion with tendency to tan rather
than burn
- Aged <40 years
|
Annual self-checks with opportunistic checks with a doctor24 |
Many patients will elect to have skin checks – frequently patients with inherently higher risk. Skin checks create an opportunity to educate the patient of preventive measures, may prompt modification of behaviours, and highlight the importance for self-examination. Patients and their family members are useful assets regarding recognition of skin cancer and suspicious lesions; the majority of melanomas are detected by patients or their family.4
GPs can adopt various ways to conduct a thorough skin examination. One method is outlined in Figure 1. The history should clearly identify all lesions of concern and changes as described by the patient. Characteristics that should raise suspicion include morphology of the lesion: Has it changed in size? Does it have an irregular shape? Has the colour changed? Other suspicious features on examination include size (diameter >7 mm), inflammation, oozing and change in sensation.
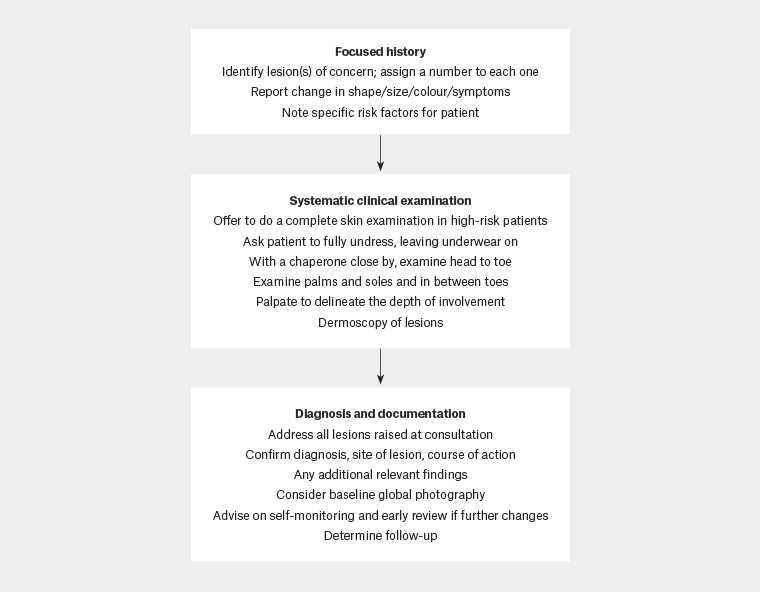
Figure 1. Approach to conducting a skin examination
Identifying the high-risk patient
There are a number of risk factors for the development of non-melanoma skin cancer (NMSC) and melanoma skin cancer (Table 1). Ultraviolet radiation is the greatest risk factor, which can be determined through detailed understanding of sun exposure behaviours, such as use of solariums, multiple episodes of sunburn and occupational history. Patients who have had one NMSC are at risk of subsequent NMSC within the next few years. It is not unusual to find more than one basal cell carcinoma (BCC) in a fair-skinned individual who has spent a lot of time outdoors; the key is to examine closely for the second or third BCC. Age and sex are also relevant considerations as risk is heightened with increasing age because of cumulative sun exposure and skin cancers are more common in males.2
Certain genetic disorders are implicated in higher risk for skin cancers, which, although they may be rarer and subtler, should be noted. People with albinism whose relatives are dark skinned are at increased risk of BCC,5 and certain genetic syndromes such as xeroderma pigmentosum are associated with a 10,000-fold increased risk of NMSC and a 2000-fold increased risk of melanoma under the age of 20.6 Younger age of NMSC presentation should raise the suspicion of an underlying genetic condition and may require independent investigation.
Renal transplant patients are at risk of NMSC and this cumulative risk increases with duration of immunosuppression, from 29.1% at <5 years to 82.1% after 20 years of immunosuppression.7 The ratio of squamous cell carcinoma (SCC) to BCC is reported to reverse after transplantation, from 1:3.7 pre-transplant to 2:1 post-transplant,7 and notably the clinical appearance of lesions may not be entirely characteristic.8 Having a lower threshold for biopsying is advised in this group.
High-risk patients should be advised to routinely perform self-examinations every 3–4 months, in conjunction with 6–12-monthly skin checks with a doctor.9 Even those deemed at average-to-low risk should still be encouraged to undertake self-checks annually and to seek their doctors’ help should they be concerned. Such practices assist in changing culture and attitudes towards skin cancer and its detection. All patients, regardless of risk, should be advised on prevention.
The role of imaging and mole surveillance
Dermatoscopes are a useful tool that can lead to improved diagnosis of melanoma and other skin malignancies. While some lesions can be relatively easily identified macroscopically, many require use of a dermatoscope for closer inspection. Efficacy is largely related to user skill. Given the scope of skin cancer in general practice, it is within reach of interested practitioners. In primary care, experience using dermatoscopy correlates with improvements for sensitivity of melanoma diagnosis.10,11 The ‘chaos and clues’ algorithm reported by Rosendahl et al allows for a structured framework in approaching diagnosis.12
Mole mapping/Vectra WB360 imaging
Revolutionising traditional medical photographs, advances in surveillance techniques have been introduced and are being integrated into clinical care. These include digital mole mapping and Vectra WB360 imaging. Monitoring multiple moles can be challenging and many incur subtle changes over time periods that are difficult to accurately record. The introduction of highly sophisticated technology provides whole-body photography producing both macro images and detailed dermatoscopic images of identified skin targets (Figure 2). Neither tool is offered in isolation but forms an adjunct to the full skin examination. Images are repeated and compared at 6–12-monthly intervals. Patients can also be provided with a printed summarised report, which may be reassuring. Genital lesions cannot be monitored by this method. Whole-body photography methods have been demonstrated as detecting early-stage cancers, offering a greater window for intervention.13 While availability of these services may vary in different geographical locations, this technology may continue to be integrated into skin checks in the future with increased research being conducted in this area.
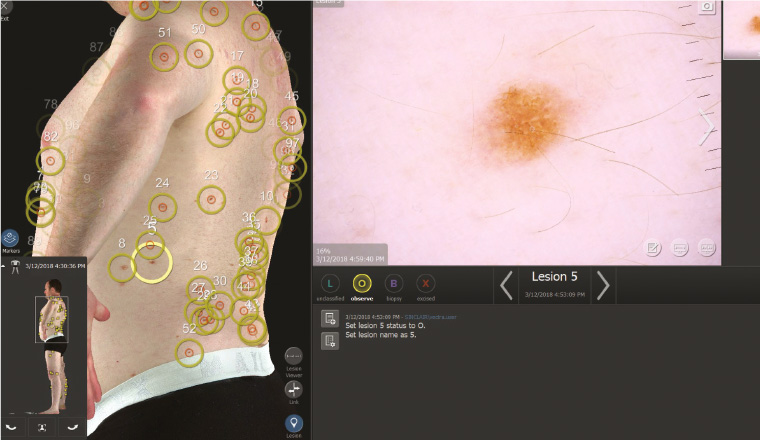
Figure 2. Vectra WB360 and dermatoscopic images
Premalignant and malignant skin lesions encountered in primary care
We offer an overview of some key features of skin malignancies and precancerous lesions that may be useful to consider when examining patients. Combining the systematic approach provided above with a general overview of common characteristics for cancerous lesions may help to improve diagnostic accuracy and prompt escalation when required.
Actinic keratosis
Actinic keratoses present as flat, red and scaly, exhibiting a sandpaper-like feel (Figure 3). Sun exposure is the greatest risk factor for development. Pigmented lesions may require a biopsy to exclude lentigo maligna. They have potential to transform into SCC, therefore some hyperkeratotic actinic keratoses may also need to be biopsied.
Treatment options include cryotherapy, which has high cure rates with a single freeze cycle of 5–10 seconds.14 Multiple actinic keratoses covering a larger surface may respond well to topical treatments including imiquimod 5% and fluorouracil 5%, as well as photodynamic therapy.
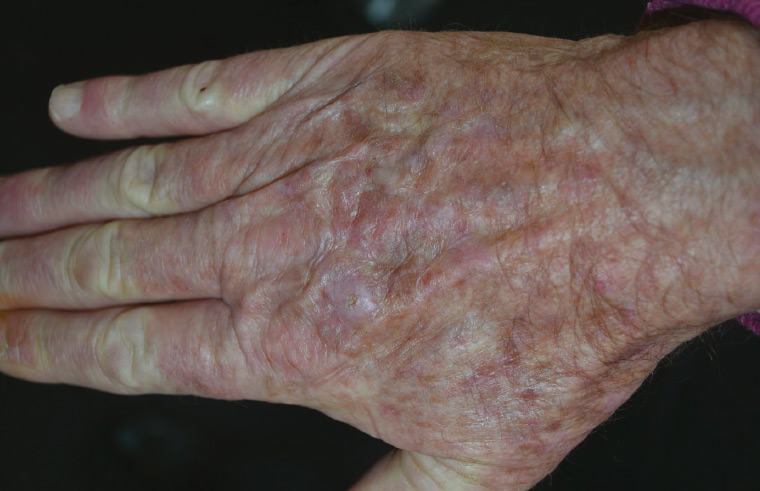
Figure 3. Hyperkeratotic actinic keratosis
Bowen’s disease
Bowen’s disease is often referred to as ‘SCC in situ’ (Figure 4). It presents as a pink, sharply defined scaly plaque with the potential to be misdiagnosed as eczema or psoriasis. It is slow growing and often presents on the lower limbs of women. Localised, biopsy-proven disease can be treated with topical therapy including imiquimod 5% and fluorouracil 5%, and photodynamic therapy. Cryotherapy is also a suitable option, using longer 30-second freeze cycles.
14
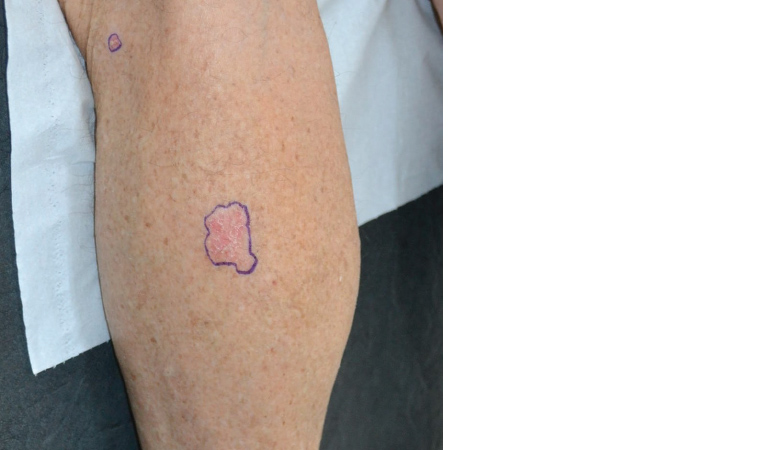
Figure 4. Bowen’s disease
Basal cell carcinoma
BCC accounts for 70% of NMSC.14 BCCs are slow growing, locally aggressive invasive epithelial tumours, with low metastatic potential. They most frequently occur on the head and neck. There are three main subtypes: nodular, superficial and morphoeic.
Nodular
Nodular BCC is the most common variant, appearing as pearly translucent nodules with characteristic rolled borders and telangiectasia (Figure 5). They frequently occur on sun-exposed sites in older, fair-skinned individuals. They may be referred to as ‘rodent ulcers’ given their tendency to ulcerate.
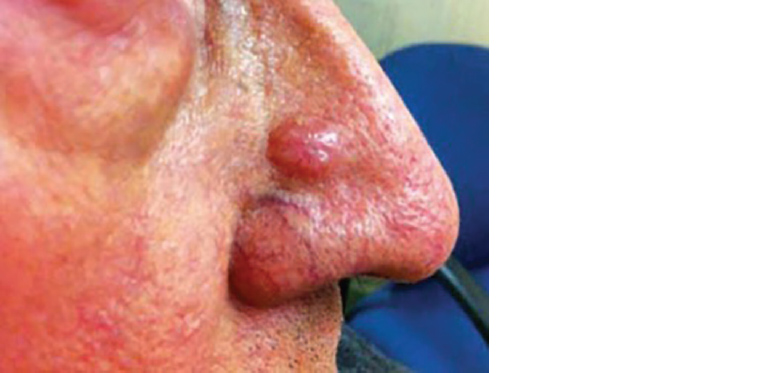
Figure 5. Nodular basal cell carcinoma
Superficial
Superficial BCCs are more common in men, and present commonly on the trunk, as a scaly, erythematous macule or patch, sometimes mimicking psoriasis or eczema. They may exhibit spotty brown pigment.
Morphoeic
Morphoeic BCCs are firm, indurated plaques with deep subclinical extension. They are waxy, with indistinct borders, and may have associated atrophy. These characteristics mean they can often be erroneously labelled as an inflammatory lesion or scar tissue. Thus, they are difficult to diagnose and are the most aggressive subtype – they may grow considerably before clinical detection.
Treatment options for basal cell carcinoma
Treatment options are dictated by tumour characteristics, size, location, pathology and patient preference. Surgical excision is recommended as first line – a simple, elliptical excision with 4–5 mm margins for most cases. The gold standard for morphoeic BCC is Mohs surgery performed by an accredited Mohs surgeon. Superficial lesions on low-risk sites such as the trunk or extremities respond well to minor surgical options such as curettage and electrodessication with low overall recurrence rates.15,16 However, it lacks histological confirmation of tumour margins, and cosmetic outcomes, particularly on the face, may be poorer when compared with excision.
Topical therapies may be appropriate as a second-line option for patients with superficial BCCs wishing to avoid surgery. Imiquimod 5% and fluorouracil 5% both elicit inflammatory responses and have been shown to achieve histological clearance rates of approximately 80% in superficial BCCs, with imiquimod yielding slightly more favourable outcomes.17
Photodynamic therapy is a non-invasive option for superficial BCCs. Results are inferior to surgery and other topical treatments.17 Radiation may be offered to elderly patients with high-risk BCC for whom surgical intervention is not appropriate.
Oral nicotinamide (vitamin B3) is an over-the-counter, safe and effective oral dietary supplement that may assist in the prevention of NMSC.18
Keratoacanthoma
Keratoacanthoma present as rapidly evolving dome-shaped nodules (up to 1–2 cm in size) with a central hyperkeratotic core and are likely a low-risk variant of SCC (Figure 6). It is unclear if this is a benign or malignant entity. Their distinctive evolution pattern is well described: a proliferative growth period lasting 12 weeks, followed by involution over three months.19 Given their clinical appearance, they are often treated as a well-differentiated SCC and should be surgically removed.
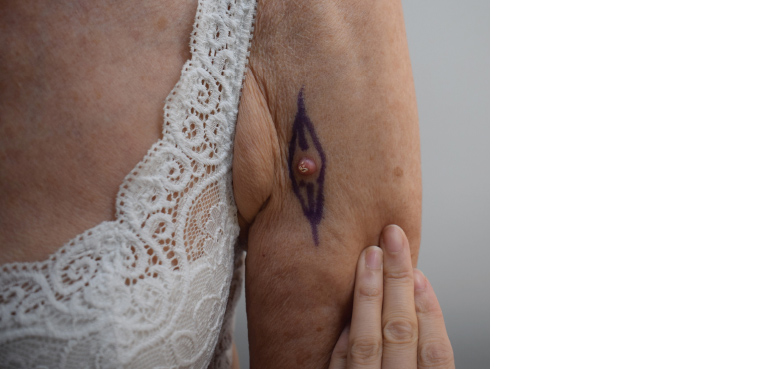
Figure 6. Keratoacanthoma
Squamous cell carcinoma
SCC is the second most common skin cancer and is a rapidly growing cutaneous cancer (Figure 7); thus, treatment should not be delayed. SCCs have a predilection for areas with heavy sun exposure, namely the head and neck; the lower lips and ears are both higher risk sites.20 Invasive SCCs extend into the dermis and are histologically characterised according to the grade of keratinocyte differentiation: well, moderate, poor or infiltrate. A well-differentiated SCC is characterised by a rapidly evolving nodule. They may bleed easily and be tender on palpation.
SCC can present in various forms – nodules, plaques, ulcers or verrucous – depending on bodily site. A non-healing ulcer on the leg of an elderly patient should raise the suspicion of an ulcerated SCC. SCC can metastasise to regional lymph nodes; hence, where indicated, lymph node examination should be part of the clinical assessment.
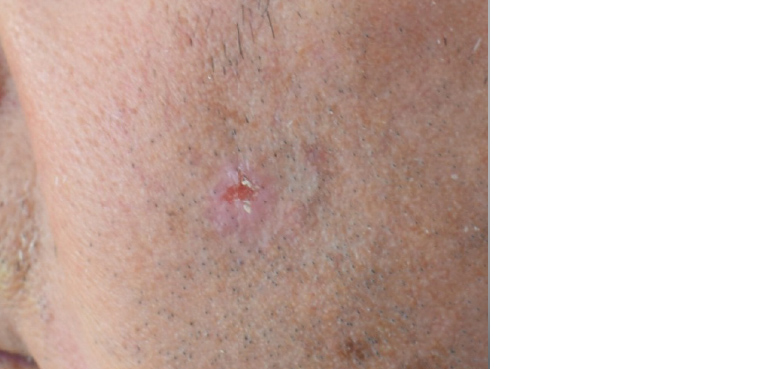
Figure 7. Squamous cell carcinoma
Malignant melanoma
There are various melanoma subtypes and a full comprehensive review is beyond the scope of this article.
Superficial spreading malignant melanoma
This is the most common and most easily treated melanoma. Most occur on the calves in women and the trunk in men and may often arise from an underlying naevus.
Nodular melanoma
This is the second most common type of melanoma and early detection can be challenging. They are often small, thick, darkly pigmented nodules or papules.
Lentigo maligna
Lentigo maligna is a melanoma in situ that develops around hair follicles and is most commonly found in older individuals with chronic sun damage (Figure 8). It develops as a slow-growing patch, often variegated with a jagged border.
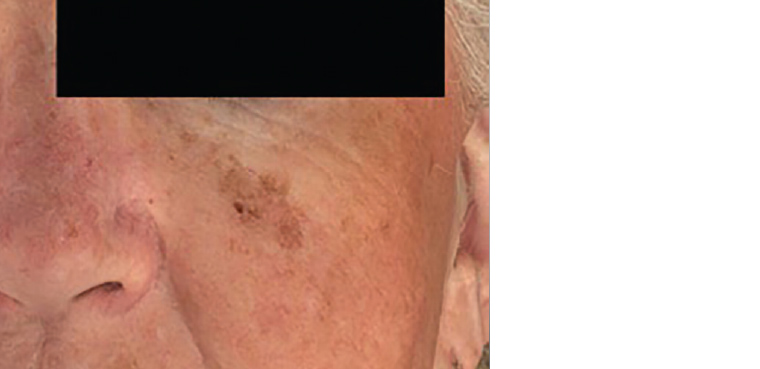
Figure 8. Lentigo maligna
Acral lentiginous melanoma
Although accounting for only 5% of melanomas, this is an important subtype to consider, given its association with darker-skinned individuals who are usually not flagged as high risk. These lesions have the tendency to arise in areas without considerable sun exposure (plantar, palmar and subungual surfaces).
Melanoma: Further issues
The occurrence of melanoma in children is rare. Amelanotic melanoma is also rare but should always be considered in a rapidly evolving pink nodule. Dermatoscopy may reveal linear irregular vessels and milky-red areas. In patients with multiple affected family members with melanoma and pancreatic cancer or personal history of several primary melanomas, a referral to a geneticist is indicated to exclude an underlying hereditary melanoma syndrome.21
Top 10 tips to avoid missing a skin cancer
- Have a lower threshold for biopsying in high-risk individuals.
- If the history does not correlate with the clinical findings of a benign lesion, photograph with supplementary dermatoscopic images and arrange a further review in three months for reassurance.
- A rapidly evolving pink nodule may be an amelanotic melanoma; these need urgent attention.
- Patients who re-present after previous reassurance are best reviewed by another experienced skin clinician for a second opinion.
- Meticulous documentation of multiple lesions can prevent pitfalls.
- Three-monthly to four-monthly reviews are advised in patients with precancerous lesions/previous history of NMSC.
- Err on the side of caution, advising early review if any lesions change prior to next visit.
- If topical treatment does not sufficiently treat a precancerous lesion, biopsy.
- If there is a rapid history of growth in a lesion clinically resembling a BCC, treat as urgent. Some SCCs can look like nodular BCCs.
- If you cannot make a diagnosis, arrange for a biopsy.
Conclusion
The ability to confidently detect and diagnose skin cancers is a skill that comes with time and experience. Lesions do not always have characteristic appearances; very occasionally there may be disparity in the history and clinical findings. Adopting a structured approach that fits in with the practicality of the clinical practice is of utmost importance to promote early detection of skin cancers. Confidence in recognising and discerning the malignant skin lesions, as well as knowledge regarding some clinical mimickers, is always helpful. Recent advances in technology including mole mapping services and Vectra WB360 serve as highly sophisticated adjuncts to the traditional skin examination. These promote closer surveillance to again facilitate early skin cancer detection. In spite of this, there will be times when clinical uncertainty will occur. In these circumstances, the options are straightforward – arrange a biopsy for histopathological confirmation or refer to dermatology.